360185
Figure shows a horizontal cylindrical container of length \(30\,cm\), which is partitioned by a tight-fitting separator. The separator is diathermic but conducts heat very slowly. Initially the separator is in the state shown in figure. The temperature of left part of cylinder is \(100\,K\) and that on right part is \(400\,K\). Initially the separator is in equilibrium. As heat is conducted from right to left part, separator displaces to the right. Find the displacement of separator when two gases on the parts of cylinder are in thermal equilibrium is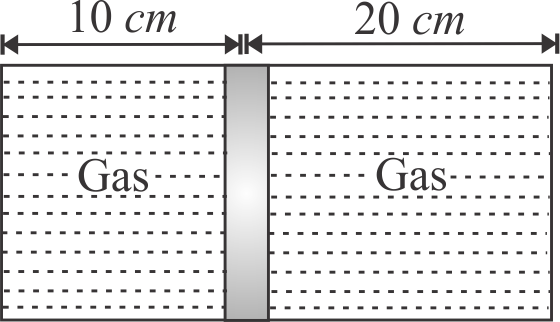
360185
Figure shows a horizontal cylindrical container of length \(30\,cm\), which is partitioned by a tight-fitting separator. The separator is diathermic but conducts heat very slowly. Initially the separator is in the state shown in figure. The temperature of left part of cylinder is \(100\,K\) and that on right part is \(400\,K\). Initially the separator is in equilibrium. As heat is conducted from right to left part, separator displaces to the right. Find the displacement of separator when two gases on the parts of cylinder are in thermal equilibrium is
360185
Figure shows a horizontal cylindrical container of length \(30\,cm\), which is partitioned by a tight-fitting separator. The separator is diathermic but conducts heat very slowly. Initially the separator is in the state shown in figure. The temperature of left part of cylinder is \(100\,K\) and that on right part is \(400\,K\). Initially the separator is in equilibrium. As heat is conducted from right to left part, separator displaces to the right. Find the displacement of separator when two gases on the parts of cylinder are in thermal equilibrium is
360185
Figure shows a horizontal cylindrical container of length \(30\,cm\), which is partitioned by a tight-fitting separator. The separator is diathermic but conducts heat very slowly. Initially the separator is in the state shown in figure. The temperature of left part of cylinder is \(100\,K\) and that on right part is \(400\,K\). Initially the separator is in equilibrium. As heat is conducted from right to left part, separator displaces to the right. Find the displacement of separator when two gases on the parts of cylinder are in thermal equilibrium is