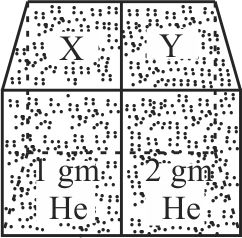
360180
Two spherical vessels of equal volume, are connected by a narrow tube. The apparatus contains an ideal gas at one atmosphere and at temperature 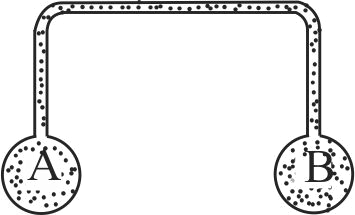
360180
Two spherical vessels of equal volume, are connected by a narrow tube. The apparatus contains an ideal gas at one atmosphere and at temperature 
360180
Two spherical vessels of equal volume, are connected by a narrow tube. The apparatus contains an ideal gas at one atmosphere and at temperature 
360180
Two spherical vessels of equal volume, are connected by a narrow tube. The apparatus contains an ideal gas at one atmosphere and at temperature 
360180
Two spherical vessels of equal volume, are connected by a narrow tube. The apparatus contains an ideal gas at one atmosphere and at temperature