360174
The two conducting cylinder-piston systems shown below are linked. Cylinder 1 is filled with a certain molar quantity of a monatomic ideal gas, and cylinder 2 is filled with an equal molar quantity of a diatomic ideal gas. The entire apparatus is situated inside an oven whose temperature is \(T_{a}=27^{\circ} {C}\). The cylinder volumes have the same initial value \(V_{0}=100 {cc}\). When the oven temperature is slowly raised to \(T_{b}=127^{\circ} {C}\). What is the volume change \(\Delta V\) of cylinder 1 ?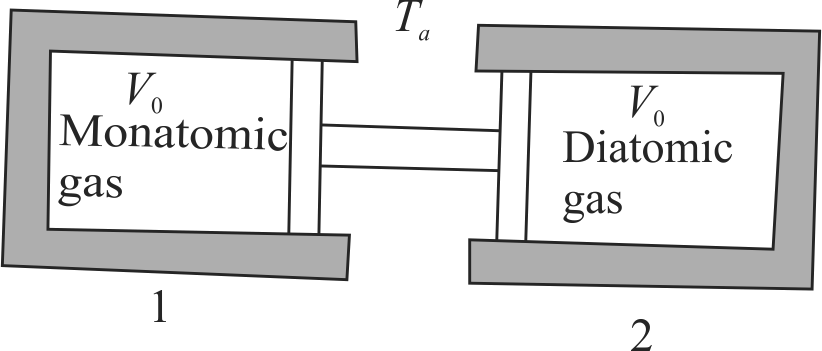
360176 Two identical glass bulbs, connected by a thin glass tube, are filled with a monoatomic gas at \(S.T.P.\) One of the bulbs is placed in a vessel containing ice while other is placed in a hot bath. The pressure of the gas increases to \(1.5\) times the initial pressure. What will be the temperature of the hot bath
360174
The two conducting cylinder-piston systems shown below are linked. Cylinder 1 is filled with a certain molar quantity of a monatomic ideal gas, and cylinder 2 is filled with an equal molar quantity of a diatomic ideal gas. The entire apparatus is situated inside an oven whose temperature is \(T_{a}=27^{\circ} {C}\). The cylinder volumes have the same initial value \(V_{0}=100 {cc}\). When the oven temperature is slowly raised to \(T_{b}=127^{\circ} {C}\). What is the volume change \(\Delta V\) of cylinder 1 ?
360176 Two identical glass bulbs, connected by a thin glass tube, are filled with a monoatomic gas at \(S.T.P.\) One of the bulbs is placed in a vessel containing ice while other is placed in a hot bath. The pressure of the gas increases to \(1.5\) times the initial pressure. What will be the temperature of the hot bath
360174
The two conducting cylinder-piston systems shown below are linked. Cylinder 1 is filled with a certain molar quantity of a monatomic ideal gas, and cylinder 2 is filled with an equal molar quantity of a diatomic ideal gas. The entire apparatus is situated inside an oven whose temperature is \(T_{a}=27^{\circ} {C}\). The cylinder volumes have the same initial value \(V_{0}=100 {cc}\). When the oven temperature is slowly raised to \(T_{b}=127^{\circ} {C}\). What is the volume change \(\Delta V\) of cylinder 1 ?
360176 Two identical glass bulbs, connected by a thin glass tube, are filled with a monoatomic gas at \(S.T.P.\) One of the bulbs is placed in a vessel containing ice while other is placed in a hot bath. The pressure of the gas increases to \(1.5\) times the initial pressure. What will be the temperature of the hot bath
360174
The two conducting cylinder-piston systems shown below are linked. Cylinder 1 is filled with a certain molar quantity of a monatomic ideal gas, and cylinder 2 is filled with an equal molar quantity of a diatomic ideal gas. The entire apparatus is situated inside an oven whose temperature is \(T_{a}=27^{\circ} {C}\). The cylinder volumes have the same initial value \(V_{0}=100 {cc}\). When the oven temperature is slowly raised to \(T_{b}=127^{\circ} {C}\). What is the volume change \(\Delta V\) of cylinder 1 ?
360176 Two identical glass bulbs, connected by a thin glass tube, are filled with a monoatomic gas at \(S.T.P.\) One of the bulbs is placed in a vessel containing ice while other is placed in a hot bath. The pressure of the gas increases to \(1.5\) times the initial pressure. What will be the temperature of the hot bath
