360170
Two glass spheres of equal volume are connected by a small tube containing a small amount of mercury as shown in figure. The spheres are sealed at \(20^\circ C\) with exactly 1 litre of air in each side. If the cross sectional area of tube is \(5\,m{m^2}\) how far will the mercury be displaced if the temperature of one sphere is raised by \(0^\circ C\) while the other is maintained at \(20^\circ C\)
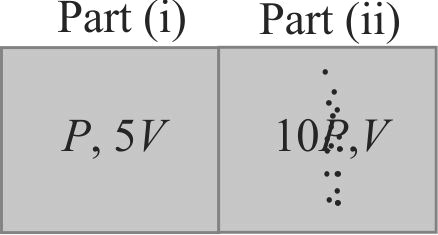
360171 A closed gas cylinder is divided into two parts by a piston held tight. The pressure and volume of gas in two parts respectively are \((P,5{\mkern 1mu} {\mkern 1mu} V)\) and \((10{\rm{ }}P,{\rm{ }}V)\). If now the piston is left free and the system undergoes isothermal process, then the volume of the gas in two parts respectively are
360170
Two glass spheres of equal volume are connected by a small tube containing a small amount of mercury as shown in figure. The spheres are sealed at \(20^\circ C\) with exactly 1 litre of air in each side. If the cross sectional area of tube is \(5\,m{m^2}\) how far will the mercury be displaced if the temperature of one sphere is raised by \(0^\circ C\) while the other is maintained at \(20^\circ C\)
360171 A closed gas cylinder is divided into two parts by a piston held tight. The pressure and volume of gas in two parts respectively are \((P,5{\mkern 1mu} {\mkern 1mu} V)\) and \((10{\rm{ }}P,{\rm{ }}V)\). If now the piston is left free and the system undergoes isothermal process, then the volume of the gas in two parts respectively are
360170
Two glass spheres of equal volume are connected by a small tube containing a small amount of mercury as shown in figure. The spheres are sealed at \(20^\circ C\) with exactly 1 litre of air in each side. If the cross sectional area of tube is \(5\,m{m^2}\) how far will the mercury be displaced if the temperature of one sphere is raised by \(0^\circ C\) while the other is maintained at \(20^\circ C\)
360171 A closed gas cylinder is divided into two parts by a piston held tight. The pressure and volume of gas in two parts respectively are \((P,5{\mkern 1mu} {\mkern 1mu} V)\) and \((10{\rm{ }}P,{\rm{ }}V)\). If now the piston is left free and the system undergoes isothermal process, then the volume of the gas in two parts respectively are
360170
Two glass spheres of equal volume are connected by a small tube containing a small amount of mercury as shown in figure. The spheres are sealed at \(20^\circ C\) with exactly 1 litre of air in each side. If the cross sectional area of tube is \(5\,m{m^2}\) how far will the mercury be displaced if the temperature of one sphere is raised by \(0^\circ C\) while the other is maintained at \(20^\circ C\)
360171 A closed gas cylinder is divided into two parts by a piston held tight. The pressure and volume of gas in two parts respectively are \((P,5{\mkern 1mu} {\mkern 1mu} V)\) and \((10{\rm{ }}P,{\rm{ }}V)\). If now the piston is left free and the system undergoes isothermal process, then the volume of the gas in two parts respectively are