360186 Two closed containers of equal volume of air are initially at \(1.05 \times 10^{5} {~Pa}\), and 300 \(K\) temperature. If the containers are connected by a narrow tube and one container is maintained at 300 \(K\) temperature and other at 400 \(K\) temperature. Find the final pressure in the containers.
360187
In the given figure a glass tube lies horizontally with the middle \(20\,\,cm\) containing mercury. The two ends of the tube contains air at \(27^\circ C\) and at a pressure \(76 \,\,cm\) of mercury. Now the air column on one side is maintained at \(0^\circ C\) and the other side is maintained at \(127^\circ C\). Find the new length of the air column on the cooler side. Neglect the changes in the volume of mercury and of the glass.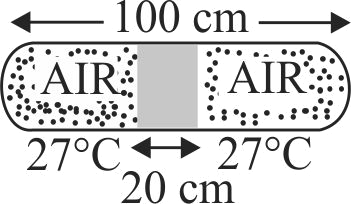
360186 Two closed containers of equal volume of air are initially at \(1.05 \times 10^{5} {~Pa}\), and 300 \(K\) temperature. If the containers are connected by a narrow tube and one container is maintained at 300 \(K\) temperature and other at 400 \(K\) temperature. Find the final pressure in the containers.
360187
In the given figure a glass tube lies horizontally with the middle \(20\,\,cm\) containing mercury. The two ends of the tube contains air at \(27^\circ C\) and at a pressure \(76 \,\,cm\) of mercury. Now the air column on one side is maintained at \(0^\circ C\) and the other side is maintained at \(127^\circ C\). Find the new length of the air column on the cooler side. Neglect the changes in the volume of mercury and of the glass.
360186 Two closed containers of equal volume of air are initially at \(1.05 \times 10^{5} {~Pa}\), and 300 \(K\) temperature. If the containers are connected by a narrow tube and one container is maintained at 300 \(K\) temperature and other at 400 \(K\) temperature. Find the final pressure in the containers.
360187
In the given figure a glass tube lies horizontally with the middle \(20\,\,cm\) containing mercury. The two ends of the tube contains air at \(27^\circ C\) and at a pressure \(76 \,\,cm\) of mercury. Now the air column on one side is maintained at \(0^\circ C\) and the other side is maintained at \(127^\circ C\). Find the new length of the air column on the cooler side. Neglect the changes in the volume of mercury and of the glass.
360186 Two closed containers of equal volume of air are initially at \(1.05 \times 10^{5} {~Pa}\), and 300 \(K\) temperature. If the containers are connected by a narrow tube and one container is maintained at 300 \(K\) temperature and other at 400 \(K\) temperature. Find the final pressure in the containers.
360187
In the given figure a glass tube lies horizontally with the middle \(20\,\,cm\) containing mercury. The two ends of the tube contains air at \(27^\circ C\) and at a pressure \(76 \,\,cm\) of mercury. Now the air column on one side is maintained at \(0^\circ C\) and the other side is maintained at \(127^\circ C\). Find the new length of the air column on the cooler side. Neglect the changes in the volume of mercury and of the glass.
