360190 Two identical containers joined by a small pipe initially contain the same gas at pressure \(p_{0}\) and absolute temperature \(T_{0}\). One container is now maintained at the same temperature while the other is heated to \(2 T_{0}\). The common pressure of the gases will be
360191
A horizontal uniform glass tube of \(100\,\,cm\), length sealed at both ends contain \(10\,\,cm\) mercury column in the middle. The temperature and pressure of air on either side of mercury column are respectively \(81^\circ C\) and \(76 \,\,cm\) of mercury. If the air column at one end is kept at \(0^\circ C\) and the other end at \(273^\circ C\), the pressure of air which is at \(0^\circ C\) is (in \(cm\) of \(Hg)\)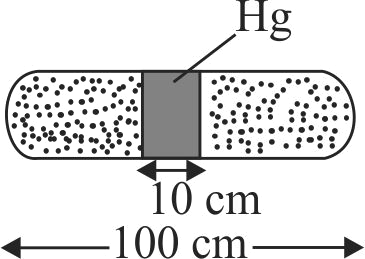
360193 Three perfect gases at absolute temperatures \(T_{1}, T_{2}\) and \(T_{3}\) are mixed. The masses of molecules are \(m_{1}, m_{2}\) and \(m_{3}\) and number of molecules are \(n_{1}, n_{2}\) and \(n_{3}\) respectively. Assuming no loss of energy, the final temperature of the mixture is:
360190 Two identical containers joined by a small pipe initially contain the same gas at pressure \(p_{0}\) and absolute temperature \(T_{0}\). One container is now maintained at the same temperature while the other is heated to \(2 T_{0}\). The common pressure of the gases will be
360191
A horizontal uniform glass tube of \(100\,\,cm\), length sealed at both ends contain \(10\,\,cm\) mercury column in the middle. The temperature and pressure of air on either side of mercury column are respectively \(81^\circ C\) and \(76 \,\,cm\) of mercury. If the air column at one end is kept at \(0^\circ C\) and the other end at \(273^\circ C\), the pressure of air which is at \(0^\circ C\) is (in \(cm\) of \(Hg)\)
360193 Three perfect gases at absolute temperatures \(T_{1}, T_{2}\) and \(T_{3}\) are mixed. The masses of molecules are \(m_{1}, m_{2}\) and \(m_{3}\) and number of molecules are \(n_{1}, n_{2}\) and \(n_{3}\) respectively. Assuming no loss of energy, the final temperature of the mixture is:
360190 Two identical containers joined by a small pipe initially contain the same gas at pressure \(p_{0}\) and absolute temperature \(T_{0}\). One container is now maintained at the same temperature while the other is heated to \(2 T_{0}\). The common pressure of the gases will be
360191
A horizontal uniform glass tube of \(100\,\,cm\), length sealed at both ends contain \(10\,\,cm\) mercury column in the middle. The temperature and pressure of air on either side of mercury column are respectively \(81^\circ C\) and \(76 \,\,cm\) of mercury. If the air column at one end is kept at \(0^\circ C\) and the other end at \(273^\circ C\), the pressure of air which is at \(0^\circ C\) is (in \(cm\) of \(Hg)\)
360193 Three perfect gases at absolute temperatures \(T_{1}, T_{2}\) and \(T_{3}\) are mixed. The masses of molecules are \(m_{1}, m_{2}\) and \(m_{3}\) and number of molecules are \(n_{1}, n_{2}\) and \(n_{3}\) respectively. Assuming no loss of energy, the final temperature of the mixture is:
360190 Two identical containers joined by a small pipe initially contain the same gas at pressure \(p_{0}\) and absolute temperature \(T_{0}\). One container is now maintained at the same temperature while the other is heated to \(2 T_{0}\). The common pressure of the gases will be
360191
A horizontal uniform glass tube of \(100\,\,cm\), length sealed at both ends contain \(10\,\,cm\) mercury column in the middle. The temperature and pressure of air on either side of mercury column are respectively \(81^\circ C\) and \(76 \,\,cm\) of mercury. If the air column at one end is kept at \(0^\circ C\) and the other end at \(273^\circ C\), the pressure of air which is at \(0^\circ C\) is (in \(cm\) of \(Hg)\)
360193 Three perfect gases at absolute temperatures \(T_{1}, T_{2}\) and \(T_{3}\) are mixed. The masses of molecules are \(m_{1}, m_{2}\) and \(m_{3}\) and number of molecules are \(n_{1}, n_{2}\) and \(n_{3}\) respectively. Assuming no loss of energy, the final temperature of the mixture is:
