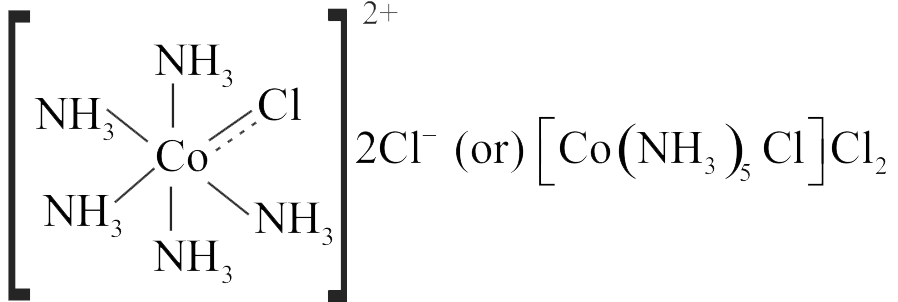322359
The correct order of the stoichiometries of \(\mathrm{AgCl}\) formed when \(\mathrm{AgNO}_{3}\) in excess is treated with the complexs:
\(\mathrm{CoCl}_{3} .6 \mathrm{NH}_{3}, \mathrm{CoCl}_{3} .5 \mathrm{NH}_{3}, \mathrm{CoCl}_{3} .4 \mathrm{NH}_{3}\) respectively is :-
322359
The correct order of the stoichiometries of \(\mathrm{AgCl}\) formed when \(\mathrm{AgNO}_{3}\) in excess is treated with the complexs:
\(\mathrm{CoCl}_{3} .6 \mathrm{NH}_{3}, \mathrm{CoCl}_{3} .5 \mathrm{NH}_{3}, \mathrm{CoCl}_{3} .4 \mathrm{NH}_{3}\) respectively is :-
322359
The correct order of the stoichiometries of \(\mathrm{AgCl}\) formed when \(\mathrm{AgNO}_{3}\) in excess is treated with the complexs:
\(\mathrm{CoCl}_{3} .6 \mathrm{NH}_{3}, \mathrm{CoCl}_{3} .5 \mathrm{NH}_{3}, \mathrm{CoCl}_{3} .4 \mathrm{NH}_{3}\) respectively is :-
322359
The correct order of the stoichiometries of \(\mathrm{AgCl}\) formed when \(\mathrm{AgNO}_{3}\) in excess is treated with the complexs:
\(\mathrm{CoCl}_{3} .6 \mathrm{NH}_{3}, \mathrm{CoCl}_{3} .5 \mathrm{NH}_{3}, \mathrm{CoCl}_{3} .4 \mathrm{NH}_{3}\) respectively is :-
322359
The correct order of the stoichiometries of \(\mathrm{AgCl}\) formed when \(\mathrm{AgNO}_{3}\) in excess is treated with the complexs:
\(\mathrm{CoCl}_{3} .6 \mathrm{NH}_{3}, \mathrm{CoCl}_{3} .5 \mathrm{NH}_{3}, \mathrm{CoCl}_{3} .4 \mathrm{NH}_{3}\) respectively is :-