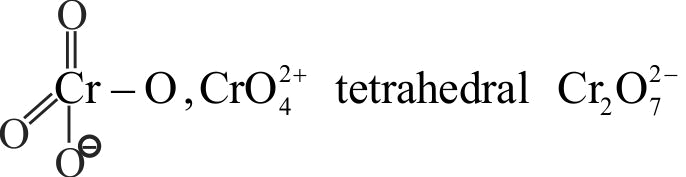
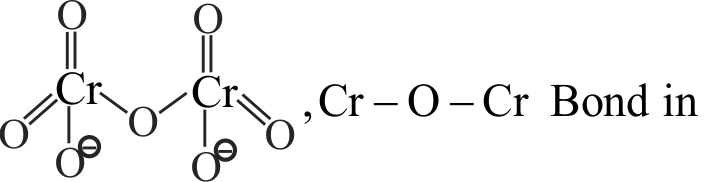
321294 A white crystalline salt A reacts with dilute \(\mathrm{\mathrm{HCl}}\) to liberate a suffocating gas B and also forms a yellow precipitate. The gas \(\mathrm{\mathrm{B}}\) turns potassium dichromate acidified with dilute \(\mathrm{\mathrm{H}_{2} \mathrm{SO}_{4}}\) to a green coloured solution C. A, B and C are respectively
321294 A white crystalline salt A reacts with dilute \(\mathrm{\mathrm{HCl}}\) to liberate a suffocating gas B and also forms a yellow precipitate. The gas \(\mathrm{\mathrm{B}}\) turns potassium dichromate acidified with dilute \(\mathrm{\mathrm{H}_{2} \mathrm{SO}_{4}}\) to a green coloured solution C. A, B and C are respectively
321294 A white crystalline salt A reacts with dilute \(\mathrm{\mathrm{HCl}}\) to liberate a suffocating gas B and also forms a yellow precipitate. The gas \(\mathrm{\mathrm{B}}\) turns potassium dichromate acidified with dilute \(\mathrm{\mathrm{H}_{2} \mathrm{SO}_{4}}\) to a green coloured solution C. A, B and C are respectively
321294 A white crystalline salt A reacts with dilute \(\mathrm{\mathrm{HCl}}\) to liberate a suffocating gas B and also forms a yellow precipitate. The gas \(\mathrm{\mathrm{B}}\) turns potassium dichromate acidified with dilute \(\mathrm{\mathrm{H}_{2} \mathrm{SO}_{4}}\) to a green coloured solution C. A, B and C are respectively
321294 A white crystalline salt A reacts with dilute \(\mathrm{\mathrm{HCl}}\) to liberate a suffocating gas B and also forms a yellow precipitate. The gas \(\mathrm{\mathrm{B}}\) turns potassium dichromate acidified with dilute \(\mathrm{\mathrm{H}_{2} \mathrm{SO}_{4}}\) to a green coloured solution C. A, B and C are respectively