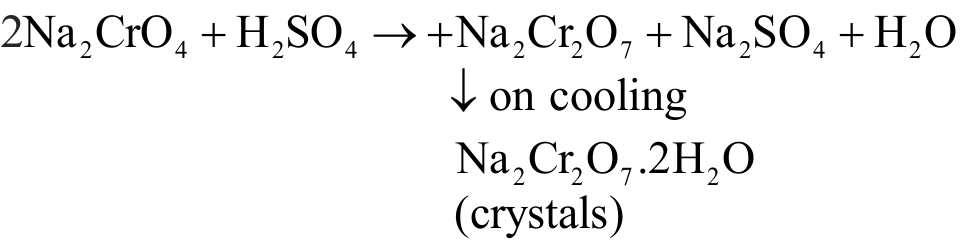Explanation:
\(4 \mathrm{FeCr}_{2} \mathrm{O}_{4}+8 \mathrm{Na}_{2} \mathrm{CO}_{3}+7 \mathrm{O}_{2} \rightarrow\)
\(\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,8{\text{N}}{{\text{a}}_2}{\text{Cr}}{{\text{O}}_4} + 2{\text{F}}{{\text{e}}_2}{{\text{O}}_3} + 8{\text{C}}{{\text{O}}_2}\)