321286
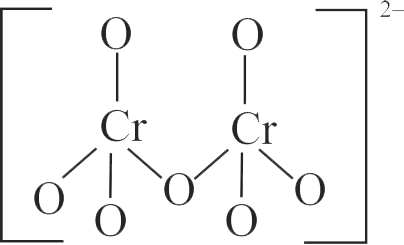
In acidic medium, \({\mathrm{\mathrm{K}_{2} \mathrm{Cr}_{2} \mathrm{O}_{7}}}\) shows oxidising action as represented in the half reaction
\({\mathrm{\mathrm{Cr}_{2} \mathrm{O}_{7}^{2-}+\mathrm{XH}^{+}+\mathrm{Ye}^{-} \rightarrow 2 \mathrm{~A}+\mathrm{ZH}_{2} \mathrm{O}}}\)
\({\mathrm{\mathrm{X}, \mathrm{Y}, \mathrm{Z}}}\) and A respectively are
321286
In acidic medium, \({\mathrm{\mathrm{K}_{2} \mathrm{Cr}_{2} \mathrm{O}_{7}}}\) shows oxidising action as represented in the half reaction
\({\mathrm{\mathrm{Cr}_{2} \mathrm{O}_{7}^{2-}+\mathrm{XH}^{+}+\mathrm{Ye}^{-} \rightarrow 2 \mathrm{~A}+\mathrm{ZH}_{2} \mathrm{O}}}\)
\({\mathrm{\mathrm{X}, \mathrm{Y}, \mathrm{Z}}}\) and A respectively are
321286
In acidic medium, \({\mathrm{\mathrm{K}_{2} \mathrm{Cr}_{2} \mathrm{O}_{7}}}\) shows oxidising action as represented in the half reaction
\({\mathrm{\mathrm{Cr}_{2} \mathrm{O}_{7}^{2-}+\mathrm{XH}^{+}+\mathrm{Ye}^{-} \rightarrow 2 \mathrm{~A}+\mathrm{ZH}_{2} \mathrm{O}}}\)
\({\mathrm{\mathrm{X}, \mathrm{Y}, \mathrm{Z}}}\) and A respectively are
321286
In acidic medium, \({\mathrm{\mathrm{K}_{2} \mathrm{Cr}_{2} \mathrm{O}_{7}}}\) shows oxidising action as represented in the half reaction
\({\mathrm{\mathrm{Cr}_{2} \mathrm{O}_{7}^{2-}+\mathrm{XH}^{+}+\mathrm{Ye}^{-} \rightarrow 2 \mathrm{~A}+\mathrm{ZH}_{2} \mathrm{O}}}\)
\({\mathrm{\mathrm{X}, \mathrm{Y}, \mathrm{Z}}}\) and A respectively are