314914
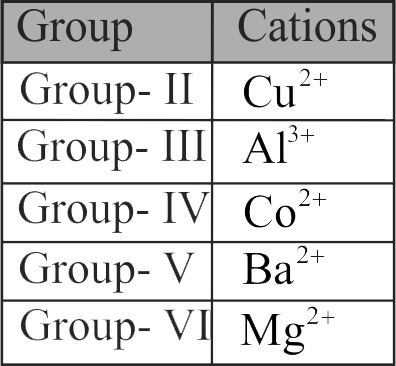
Given below are certain cations. Using inorganic qualitative analysis, arrange them in increasing group number from 0 to VI.
\[\begin{array}{l}
{\rm{(A)A}}{{\rm{l}}^{{\rm{3 + }}}}\quad {\rm{(B)C}}{{\rm{u}}^{{\rm{2 + }}}}\quad \\
{\rm{(C)B}}{{\rm{a}}^{{\rm{2 + }}}}\quad {\rm{(D)C}}{{\rm{o}}^{{\rm{2 + }}}}\quad \\
{\rm{(E)M}}{{\rm{g}}^{{\rm{2 + }}}}
\end{array}\]
Choose the correct answer from the options given below.
314916
Equal volumes of several solutions at 0.10 molar concentration are mixed. The chart below indicates the interactions that took place between these solutions. What is the formula of the hypothetical compound that has low solubility?
\[\begin{array}{*{20}{c}}
{}&{{\rm{CX}}}&{{\rm{BY}}}&{{\rm{DY}}}\\
{{\rm{AX}}}&{\rm{ \ldots }}&{{\rm{Ppt}}}&{\rm{ \ldots }}\\
{{\rm{DY}}}&{\rm{ \ldots }}&{{\rm{ \ldots }}{\rm{.}}}&{}\\
{{\rm{CX}}}&{\rm{ \ldots }}&{{\rm{Ppt}}}&{}
\end{array}\]
314917 Solid \(\mathrm{AgNO}_{3}\) is added to a solution which is 0.1 \(\mathrm{M}\) in \(\mathrm{CrO}_{4}^{2-} \cdot \mathrm{K}_{\text {sp }}\) values of \(\mathrm{AgCl}\) and \(\mathrm{AgCrO}_{4}\) are \(1.7 \times 10^{-10}\) and \(1.9 \times 10^{-12}\) respectively. The concentration of \(\mathrm{Cl}^{-}\)when \(\mathrm{Ag}_{2} \mathrm{CrO}_{4}\) starts precipitating will be:
314914
Given below are certain cations. Using inorganic qualitative analysis, arrange them in increasing group number from 0 to VI.
\[\begin{array}{l}
{\rm{(A)A}}{{\rm{l}}^{{\rm{3 + }}}}\quad {\rm{(B)C}}{{\rm{u}}^{{\rm{2 + }}}}\quad \\
{\rm{(C)B}}{{\rm{a}}^{{\rm{2 + }}}}\quad {\rm{(D)C}}{{\rm{o}}^{{\rm{2 + }}}}\quad \\
{\rm{(E)M}}{{\rm{g}}^{{\rm{2 + }}}}
\end{array}\]
Choose the correct answer from the options given below.
314916
Equal volumes of several solutions at 0.10 molar concentration are mixed. The chart below indicates the interactions that took place between these solutions. What is the formula of the hypothetical compound that has low solubility?
\[\begin{array}{*{20}{c}}
{}&{{\rm{CX}}}&{{\rm{BY}}}&{{\rm{DY}}}\\
{{\rm{AX}}}&{\rm{ \ldots }}&{{\rm{Ppt}}}&{\rm{ \ldots }}\\
{{\rm{DY}}}&{\rm{ \ldots }}&{{\rm{ \ldots }}{\rm{.}}}&{}\\
{{\rm{CX}}}&{\rm{ \ldots }}&{{\rm{Ppt}}}&{}
\end{array}\]
314917 Solid \(\mathrm{AgNO}_{3}\) is added to a solution which is 0.1 \(\mathrm{M}\) in \(\mathrm{CrO}_{4}^{2-} \cdot \mathrm{K}_{\text {sp }}\) values of \(\mathrm{AgCl}\) and \(\mathrm{AgCrO}_{4}\) are \(1.7 \times 10^{-10}\) and \(1.9 \times 10^{-12}\) respectively. The concentration of \(\mathrm{Cl}^{-}\)when \(\mathrm{Ag}_{2} \mathrm{CrO}_{4}\) starts precipitating will be:
314914
Given below are certain cations. Using inorganic qualitative analysis, arrange them in increasing group number from 0 to VI.
\[\begin{array}{l}
{\rm{(A)A}}{{\rm{l}}^{{\rm{3 + }}}}\quad {\rm{(B)C}}{{\rm{u}}^{{\rm{2 + }}}}\quad \\
{\rm{(C)B}}{{\rm{a}}^{{\rm{2 + }}}}\quad {\rm{(D)C}}{{\rm{o}}^{{\rm{2 + }}}}\quad \\
{\rm{(E)M}}{{\rm{g}}^{{\rm{2 + }}}}
\end{array}\]
Choose the correct answer from the options given below.
314916
Equal volumes of several solutions at 0.10 molar concentration are mixed. The chart below indicates the interactions that took place between these solutions. What is the formula of the hypothetical compound that has low solubility?
\[\begin{array}{*{20}{c}}
{}&{{\rm{CX}}}&{{\rm{BY}}}&{{\rm{DY}}}\\
{{\rm{AX}}}&{\rm{ \ldots }}&{{\rm{Ppt}}}&{\rm{ \ldots }}\\
{{\rm{DY}}}&{\rm{ \ldots }}&{{\rm{ \ldots }}{\rm{.}}}&{}\\
{{\rm{CX}}}&{\rm{ \ldots }}&{{\rm{Ppt}}}&{}
\end{array}\]
314917 Solid \(\mathrm{AgNO}_{3}\) is added to a solution which is 0.1 \(\mathrm{M}\) in \(\mathrm{CrO}_{4}^{2-} \cdot \mathrm{K}_{\text {sp }}\) values of \(\mathrm{AgCl}\) and \(\mathrm{AgCrO}_{4}\) are \(1.7 \times 10^{-10}\) and \(1.9 \times 10^{-12}\) respectively. The concentration of \(\mathrm{Cl}^{-}\)when \(\mathrm{Ag}_{2} \mathrm{CrO}_{4}\) starts precipitating will be:
314914
Given below are certain cations. Using inorganic qualitative analysis, arrange them in increasing group number from 0 to VI.
\[\begin{array}{l}
{\rm{(A)A}}{{\rm{l}}^{{\rm{3 + }}}}\quad {\rm{(B)C}}{{\rm{u}}^{{\rm{2 + }}}}\quad \\
{\rm{(C)B}}{{\rm{a}}^{{\rm{2 + }}}}\quad {\rm{(D)C}}{{\rm{o}}^{{\rm{2 + }}}}\quad \\
{\rm{(E)M}}{{\rm{g}}^{{\rm{2 + }}}}
\end{array}\]
Choose the correct answer from the options given below.
314916
Equal volumes of several solutions at 0.10 molar concentration are mixed. The chart below indicates the interactions that took place between these solutions. What is the formula of the hypothetical compound that has low solubility?
\[\begin{array}{*{20}{c}}
{}&{{\rm{CX}}}&{{\rm{BY}}}&{{\rm{DY}}}\\
{{\rm{AX}}}&{\rm{ \ldots }}&{{\rm{Ppt}}}&{\rm{ \ldots }}\\
{{\rm{DY}}}&{\rm{ \ldots }}&{{\rm{ \ldots }}{\rm{.}}}&{}\\
{{\rm{CX}}}&{\rm{ \ldots }}&{{\rm{Ppt}}}&{}
\end{array}\]
314917 Solid \(\mathrm{AgNO}_{3}\) is added to a solution which is 0.1 \(\mathrm{M}\) in \(\mathrm{CrO}_{4}^{2-} \cdot \mathrm{K}_{\text {sp }}\) values of \(\mathrm{AgCl}\) and \(\mathrm{AgCrO}_{4}\) are \(1.7 \times 10^{-10}\) and \(1.9 \times 10^{-12}\) respectively. The concentration of \(\mathrm{Cl}^{-}\)when \(\mathrm{Ag}_{2} \mathrm{CrO}_{4}\) starts precipitating will be: