369493 The heat of formation of \(\mathrm{\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{OH}_{(\mathrm{l})}}\) is \({\rm{ - 66}}\,{\rm{k}}\,{\rm{cal/}}\) mol. The heat of combustion of \(\mathrm{\mathrm{CH}_{3} \mathrm{OCH}_{3(\mathrm{~g})}}\) is \({\rm{ - 348}}\,{\rm{k}}\,{\rm{cal/mol}}{\rm{.}}\) \({\rm{\Delta }}{{\rm{H}}_{\rm{f}}}\) for \(\mathrm{\mathrm{H}_{2} \mathrm{O}}\) and \(\mathrm{\mathrm{CO}_{2}}\) are \({\rm{ - 68}}\)\(\mathrm{\mathrm{kcal} / \mathrm{mol}}\) and \({\rm{ - 94}}\,{\rm{k}}\,{\rm{cal/mol}}\), respectively. Then, \(\mathrm{\Delta H}\) for the isomerisation reaction \(\mathrm{\mathrm{CH}_{3} \mathrm{OCH}_{3(\mathrm{~g})}}\) is
369494
Given,
\({{\rm{N}}{{\rm{H}}_3}(\;{\rm{g}}) + 3{\rm{C}}{{\rm{l}}_2}(\;{\rm{g}})}\)\( \rightleftharpoons \)\({{\rm{NC}}{{\rm{l}}_3}(\;{\rm{g}}) + 3{\rm{HCl}}({\rm{g}});}\)
\({ - {\rm{\Delta }}{{\rm{H}}_1}}\)
\({{{\rm{N}}_2}({\rm{g}}) + 3{{\rm{H}}_2}({\rm{g}})}\)\( \rightleftharpoons \)\({2{\rm{N}}{{\rm{H}}_3}({\rm{g}});}\)
\({ - {\rm{\Delta }}{{\rm{H}}_2}}\)
\({{\rm{H}}_{\rm{2}}}{\rm{(g)}} + {\rm{C}}{{\rm{l}}_{\rm{2}}}{\rm{(g)}}\)\( \rightleftharpoons \)\(2{\rm{HCl(g)}};\)
\({\rm{\Delta }}{{\rm{H}}_{\rm{3}}}\)
The heat of formation of \(\mathrm{NCl}_{3}(\mathrm{~g})\)
in terms of \(\Delta \mathrm{H}_{1}, \Delta \mathrm{H}_{2}\) and \(\Delta \mathrm{H}_{3}\) is
369495
In the reaction,
\({\rm{S + }}\frac{{\rm{3}}}{{\rm{2}}}{{\rm{O}}_{\rm{2}}} \to {\rm{S}}{{\rm{O}}_{\rm{3}}}{\rm{ + 2xkJ}}\) and \({\rm{S}}{{\rm{O}}_{\rm{2}}}{\rm{ + }}\frac{{\rm{1}}}{{\rm{2}}}{{\rm{O}}_{\rm{2}}} \to {\rm{S}}{{\rm{O}}_{\rm{3}}}{\rm{ + ykJ}}\)
heat of formation of \(\mathrm{\mathrm{SO}_{2}}\) is
369496
Given
\({\rm{C + 2}}\;{\rm{S}} \to {\rm{C}}{{\rm{S}}_{\rm{2}}},{\Delta _{\rm{f}}}{\rm{H}}^\circ {\rm{ = + 117}}.{\rm{0}}\;{\rm{kJ}}\;{\rm{mo}}{{\rm{l}}^{{\rm{ - 1}}}}\)
\({\rm{C + }}{{\rm{O}}_{\rm{2}}} \to {\rm{C}}{{\rm{O}}_{\rm{2}}},{\Delta _{\rm{f}}}{\rm{H}}^\circ {\rm{ = - 393}}.{\rm{0}}\;{\rm{kJ}}\;{\rm{mo}}{{\rm{l}}^{{\rm{ - 1}}}}\)
\(\;{\rm{S + }}{{\rm{O}}_{\rm{2}}} \to {\rm{S}}{{\rm{O}}_{\rm{2}}},{\Delta _{\rm{f}}}{\rm{H}}^\circ {\rm{ = - 297}}.{\rm{0}}\;{\rm{kJ}}\;{\rm{mo}}{{\rm{l}}^{{\rm{ - 1}}}}\)
The heat of combustion of
\(\mathrm{\mathrm{CS}_{2}+3 \mathrm{O}_{2} \rightarrow \mathrm{CO}_{2}+2 \mathrm{SO}_{2}}\) is
369497
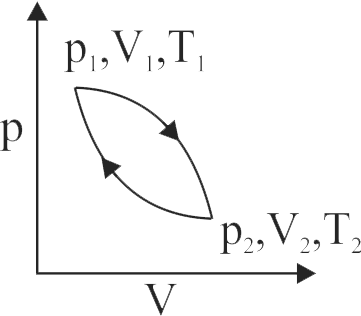
A sample of gas changes from \({{\rm{p}}_{\rm{1}}}{\rm{,}}\) \({{\rm{V}}_{\rm{1}}}\) and \({{\rm{T}}_{\rm{1}}}\) to \({{\rm{p}}_{\rm{2}}}{\rm{,}}\) \({{\rm{V}}_{\rm{2}}}\) and \({{\rm{T}}_{\rm{2}}}\) by one path and then back to \({{\rm{p}}_{\rm{1}}}{\rm{,}}\) \({{\rm{V}}_{\rm{1}}}\) and \({{\rm{T}}_{\rm{1}}}\) by another path. How many of the following must be zero for the gas in this cycle?
\(\Delta {\rm{T}},\Delta \,{\rm{p}},\Delta {\rm{V}},\,\,{\rm{q}}{\rm{.}}\,\,{\rm{W}}\) and \(\Delta \,{\rm{E}},\)
369493 The heat of formation of \(\mathrm{\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{OH}_{(\mathrm{l})}}\) is \({\rm{ - 66}}\,{\rm{k}}\,{\rm{cal/}}\) mol. The heat of combustion of \(\mathrm{\mathrm{CH}_{3} \mathrm{OCH}_{3(\mathrm{~g})}}\) is \({\rm{ - 348}}\,{\rm{k}}\,{\rm{cal/mol}}{\rm{.}}\) \({\rm{\Delta }}{{\rm{H}}_{\rm{f}}}\) for \(\mathrm{\mathrm{H}_{2} \mathrm{O}}\) and \(\mathrm{\mathrm{CO}_{2}}\) are \({\rm{ - 68}}\)\(\mathrm{\mathrm{kcal} / \mathrm{mol}}\) and \({\rm{ - 94}}\,{\rm{k}}\,{\rm{cal/mol}}\), respectively. Then, \(\mathrm{\Delta H}\) for the isomerisation reaction \(\mathrm{\mathrm{CH}_{3} \mathrm{OCH}_{3(\mathrm{~g})}}\) is
369494
Given,
\({{\rm{N}}{{\rm{H}}_3}(\;{\rm{g}}) + 3{\rm{C}}{{\rm{l}}_2}(\;{\rm{g}})}\)\( \rightleftharpoons \)\({{\rm{NC}}{{\rm{l}}_3}(\;{\rm{g}}) + 3{\rm{HCl}}({\rm{g}});}\)
\({ - {\rm{\Delta }}{{\rm{H}}_1}}\)
\({{{\rm{N}}_2}({\rm{g}}) + 3{{\rm{H}}_2}({\rm{g}})}\)\( \rightleftharpoons \)\({2{\rm{N}}{{\rm{H}}_3}({\rm{g}});}\)
\({ - {\rm{\Delta }}{{\rm{H}}_2}}\)
\({{\rm{H}}_{\rm{2}}}{\rm{(g)}} + {\rm{C}}{{\rm{l}}_{\rm{2}}}{\rm{(g)}}\)\( \rightleftharpoons \)\(2{\rm{HCl(g)}};\)
\({\rm{\Delta }}{{\rm{H}}_{\rm{3}}}\)
The heat of formation of \(\mathrm{NCl}_{3}(\mathrm{~g})\)
in terms of \(\Delta \mathrm{H}_{1}, \Delta \mathrm{H}_{2}\) and \(\Delta \mathrm{H}_{3}\) is
369495
In the reaction,
\({\rm{S + }}\frac{{\rm{3}}}{{\rm{2}}}{{\rm{O}}_{\rm{2}}} \to {\rm{S}}{{\rm{O}}_{\rm{3}}}{\rm{ + 2xkJ}}\) and \({\rm{S}}{{\rm{O}}_{\rm{2}}}{\rm{ + }}\frac{{\rm{1}}}{{\rm{2}}}{{\rm{O}}_{\rm{2}}} \to {\rm{S}}{{\rm{O}}_{\rm{3}}}{\rm{ + ykJ}}\)
heat of formation of \(\mathrm{\mathrm{SO}_{2}}\) is
369496
Given
\({\rm{C + 2}}\;{\rm{S}} \to {\rm{C}}{{\rm{S}}_{\rm{2}}},{\Delta _{\rm{f}}}{\rm{H}}^\circ {\rm{ = + 117}}.{\rm{0}}\;{\rm{kJ}}\;{\rm{mo}}{{\rm{l}}^{{\rm{ - 1}}}}\)
\({\rm{C + }}{{\rm{O}}_{\rm{2}}} \to {\rm{C}}{{\rm{O}}_{\rm{2}}},{\Delta _{\rm{f}}}{\rm{H}}^\circ {\rm{ = - 393}}.{\rm{0}}\;{\rm{kJ}}\;{\rm{mo}}{{\rm{l}}^{{\rm{ - 1}}}}\)
\(\;{\rm{S + }}{{\rm{O}}_{\rm{2}}} \to {\rm{S}}{{\rm{O}}_{\rm{2}}},{\Delta _{\rm{f}}}{\rm{H}}^\circ {\rm{ = - 297}}.{\rm{0}}\;{\rm{kJ}}\;{\rm{mo}}{{\rm{l}}^{{\rm{ - 1}}}}\)
The heat of combustion of
\(\mathrm{\mathrm{CS}_{2}+3 \mathrm{O}_{2} \rightarrow \mathrm{CO}_{2}+2 \mathrm{SO}_{2}}\) is
369497
A sample of gas changes from \({{\rm{p}}_{\rm{1}}}{\rm{,}}\) \({{\rm{V}}_{\rm{1}}}\) and \({{\rm{T}}_{\rm{1}}}\) to \({{\rm{p}}_{\rm{2}}}{\rm{,}}\) \({{\rm{V}}_{\rm{2}}}\) and \({{\rm{T}}_{\rm{2}}}\) by one path and then back to \({{\rm{p}}_{\rm{1}}}{\rm{,}}\) \({{\rm{V}}_{\rm{1}}}\) and \({{\rm{T}}_{\rm{1}}}\) by another path. How many of the following must be zero for the gas in this cycle?
\(\Delta {\rm{T}},\Delta \,{\rm{p}},\Delta {\rm{V}},\,\,{\rm{q}}{\rm{.}}\,\,{\rm{W}}\) and \(\Delta \,{\rm{E}},\)
369493 The heat of formation of \(\mathrm{\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{OH}_{(\mathrm{l})}}\) is \({\rm{ - 66}}\,{\rm{k}}\,{\rm{cal/}}\) mol. The heat of combustion of \(\mathrm{\mathrm{CH}_{3} \mathrm{OCH}_{3(\mathrm{~g})}}\) is \({\rm{ - 348}}\,{\rm{k}}\,{\rm{cal/mol}}{\rm{.}}\) \({\rm{\Delta }}{{\rm{H}}_{\rm{f}}}\) for \(\mathrm{\mathrm{H}_{2} \mathrm{O}}\) and \(\mathrm{\mathrm{CO}_{2}}\) are \({\rm{ - 68}}\)\(\mathrm{\mathrm{kcal} / \mathrm{mol}}\) and \({\rm{ - 94}}\,{\rm{k}}\,{\rm{cal/mol}}\), respectively. Then, \(\mathrm{\Delta H}\) for the isomerisation reaction \(\mathrm{\mathrm{CH}_{3} \mathrm{OCH}_{3(\mathrm{~g})}}\) is
369494
Given,
\({{\rm{N}}{{\rm{H}}_3}(\;{\rm{g}}) + 3{\rm{C}}{{\rm{l}}_2}(\;{\rm{g}})}\)\( \rightleftharpoons \)\({{\rm{NC}}{{\rm{l}}_3}(\;{\rm{g}}) + 3{\rm{HCl}}({\rm{g}});}\)
\({ - {\rm{\Delta }}{{\rm{H}}_1}}\)
\({{{\rm{N}}_2}({\rm{g}}) + 3{{\rm{H}}_2}({\rm{g}})}\)\( \rightleftharpoons \)\({2{\rm{N}}{{\rm{H}}_3}({\rm{g}});}\)
\({ - {\rm{\Delta }}{{\rm{H}}_2}}\)
\({{\rm{H}}_{\rm{2}}}{\rm{(g)}} + {\rm{C}}{{\rm{l}}_{\rm{2}}}{\rm{(g)}}\)\( \rightleftharpoons \)\(2{\rm{HCl(g)}};\)
\({\rm{\Delta }}{{\rm{H}}_{\rm{3}}}\)
The heat of formation of \(\mathrm{NCl}_{3}(\mathrm{~g})\)
in terms of \(\Delta \mathrm{H}_{1}, \Delta \mathrm{H}_{2}\) and \(\Delta \mathrm{H}_{3}\) is
369495
In the reaction,
\({\rm{S + }}\frac{{\rm{3}}}{{\rm{2}}}{{\rm{O}}_{\rm{2}}} \to {\rm{S}}{{\rm{O}}_{\rm{3}}}{\rm{ + 2xkJ}}\) and \({\rm{S}}{{\rm{O}}_{\rm{2}}}{\rm{ + }}\frac{{\rm{1}}}{{\rm{2}}}{{\rm{O}}_{\rm{2}}} \to {\rm{S}}{{\rm{O}}_{\rm{3}}}{\rm{ + ykJ}}\)
heat of formation of \(\mathrm{\mathrm{SO}_{2}}\) is
369496
Given
\({\rm{C + 2}}\;{\rm{S}} \to {\rm{C}}{{\rm{S}}_{\rm{2}}},{\Delta _{\rm{f}}}{\rm{H}}^\circ {\rm{ = + 117}}.{\rm{0}}\;{\rm{kJ}}\;{\rm{mo}}{{\rm{l}}^{{\rm{ - 1}}}}\)
\({\rm{C + }}{{\rm{O}}_{\rm{2}}} \to {\rm{C}}{{\rm{O}}_{\rm{2}}},{\Delta _{\rm{f}}}{\rm{H}}^\circ {\rm{ = - 393}}.{\rm{0}}\;{\rm{kJ}}\;{\rm{mo}}{{\rm{l}}^{{\rm{ - 1}}}}\)
\(\;{\rm{S + }}{{\rm{O}}_{\rm{2}}} \to {\rm{S}}{{\rm{O}}_{\rm{2}}},{\Delta _{\rm{f}}}{\rm{H}}^\circ {\rm{ = - 297}}.{\rm{0}}\;{\rm{kJ}}\;{\rm{mo}}{{\rm{l}}^{{\rm{ - 1}}}}\)
The heat of combustion of
\(\mathrm{\mathrm{CS}_{2}+3 \mathrm{O}_{2} \rightarrow \mathrm{CO}_{2}+2 \mathrm{SO}_{2}}\) is
369497
A sample of gas changes from \({{\rm{p}}_{\rm{1}}}{\rm{,}}\) \({{\rm{V}}_{\rm{1}}}\) and \({{\rm{T}}_{\rm{1}}}\) to \({{\rm{p}}_{\rm{2}}}{\rm{,}}\) \({{\rm{V}}_{\rm{2}}}\) and \({{\rm{T}}_{\rm{2}}}\) by one path and then back to \({{\rm{p}}_{\rm{1}}}{\rm{,}}\) \({{\rm{V}}_{\rm{1}}}\) and \({{\rm{T}}_{\rm{1}}}\) by another path. How many of the following must be zero for the gas in this cycle?
\(\Delta {\rm{T}},\Delta \,{\rm{p}},\Delta {\rm{V}},\,\,{\rm{q}}{\rm{.}}\,\,{\rm{W}}\) and \(\Delta \,{\rm{E}},\)
369493 The heat of formation of \(\mathrm{\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{OH}_{(\mathrm{l})}}\) is \({\rm{ - 66}}\,{\rm{k}}\,{\rm{cal/}}\) mol. The heat of combustion of \(\mathrm{\mathrm{CH}_{3} \mathrm{OCH}_{3(\mathrm{~g})}}\) is \({\rm{ - 348}}\,{\rm{k}}\,{\rm{cal/mol}}{\rm{.}}\) \({\rm{\Delta }}{{\rm{H}}_{\rm{f}}}\) for \(\mathrm{\mathrm{H}_{2} \mathrm{O}}\) and \(\mathrm{\mathrm{CO}_{2}}\) are \({\rm{ - 68}}\)\(\mathrm{\mathrm{kcal} / \mathrm{mol}}\) and \({\rm{ - 94}}\,{\rm{k}}\,{\rm{cal/mol}}\), respectively. Then, \(\mathrm{\Delta H}\) for the isomerisation reaction \(\mathrm{\mathrm{CH}_{3} \mathrm{OCH}_{3(\mathrm{~g})}}\) is
369494
Given,
\({{\rm{N}}{{\rm{H}}_3}(\;{\rm{g}}) + 3{\rm{C}}{{\rm{l}}_2}(\;{\rm{g}})}\)\( \rightleftharpoons \)\({{\rm{NC}}{{\rm{l}}_3}(\;{\rm{g}}) + 3{\rm{HCl}}({\rm{g}});}\)
\({ - {\rm{\Delta }}{{\rm{H}}_1}}\)
\({{{\rm{N}}_2}({\rm{g}}) + 3{{\rm{H}}_2}({\rm{g}})}\)\( \rightleftharpoons \)\({2{\rm{N}}{{\rm{H}}_3}({\rm{g}});}\)
\({ - {\rm{\Delta }}{{\rm{H}}_2}}\)
\({{\rm{H}}_{\rm{2}}}{\rm{(g)}} + {\rm{C}}{{\rm{l}}_{\rm{2}}}{\rm{(g)}}\)\( \rightleftharpoons \)\(2{\rm{HCl(g)}};\)
\({\rm{\Delta }}{{\rm{H}}_{\rm{3}}}\)
The heat of formation of \(\mathrm{NCl}_{3}(\mathrm{~g})\)
in terms of \(\Delta \mathrm{H}_{1}, \Delta \mathrm{H}_{2}\) and \(\Delta \mathrm{H}_{3}\) is
369495
In the reaction,
\({\rm{S + }}\frac{{\rm{3}}}{{\rm{2}}}{{\rm{O}}_{\rm{2}}} \to {\rm{S}}{{\rm{O}}_{\rm{3}}}{\rm{ + 2xkJ}}\) and \({\rm{S}}{{\rm{O}}_{\rm{2}}}{\rm{ + }}\frac{{\rm{1}}}{{\rm{2}}}{{\rm{O}}_{\rm{2}}} \to {\rm{S}}{{\rm{O}}_{\rm{3}}}{\rm{ + ykJ}}\)
heat of formation of \(\mathrm{\mathrm{SO}_{2}}\) is
369496
Given
\({\rm{C + 2}}\;{\rm{S}} \to {\rm{C}}{{\rm{S}}_{\rm{2}}},{\Delta _{\rm{f}}}{\rm{H}}^\circ {\rm{ = + 117}}.{\rm{0}}\;{\rm{kJ}}\;{\rm{mo}}{{\rm{l}}^{{\rm{ - 1}}}}\)
\({\rm{C + }}{{\rm{O}}_{\rm{2}}} \to {\rm{C}}{{\rm{O}}_{\rm{2}}},{\Delta _{\rm{f}}}{\rm{H}}^\circ {\rm{ = - 393}}.{\rm{0}}\;{\rm{kJ}}\;{\rm{mo}}{{\rm{l}}^{{\rm{ - 1}}}}\)
\(\;{\rm{S + }}{{\rm{O}}_{\rm{2}}} \to {\rm{S}}{{\rm{O}}_{\rm{2}}},{\Delta _{\rm{f}}}{\rm{H}}^\circ {\rm{ = - 297}}.{\rm{0}}\;{\rm{kJ}}\;{\rm{mo}}{{\rm{l}}^{{\rm{ - 1}}}}\)
The heat of combustion of
\(\mathrm{\mathrm{CS}_{2}+3 \mathrm{O}_{2} \rightarrow \mathrm{CO}_{2}+2 \mathrm{SO}_{2}}\) is
369497
A sample of gas changes from \({{\rm{p}}_{\rm{1}}}{\rm{,}}\) \({{\rm{V}}_{\rm{1}}}\) and \({{\rm{T}}_{\rm{1}}}\) to \({{\rm{p}}_{\rm{2}}}{\rm{,}}\) \({{\rm{V}}_{\rm{2}}}\) and \({{\rm{T}}_{\rm{2}}}\) by one path and then back to \({{\rm{p}}_{\rm{1}}}{\rm{,}}\) \({{\rm{V}}_{\rm{1}}}\) and \({{\rm{T}}_{\rm{1}}}\) by another path. How many of the following must be zero for the gas in this cycle?
\(\Delta {\rm{T}},\Delta \,{\rm{p}},\Delta {\rm{V}},\,\,{\rm{q}}{\rm{.}}\,\,{\rm{W}}\) and \(\Delta \,{\rm{E}},\)
369493 The heat of formation of \(\mathrm{\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{OH}_{(\mathrm{l})}}\) is \({\rm{ - 66}}\,{\rm{k}}\,{\rm{cal/}}\) mol. The heat of combustion of \(\mathrm{\mathrm{CH}_{3} \mathrm{OCH}_{3(\mathrm{~g})}}\) is \({\rm{ - 348}}\,{\rm{k}}\,{\rm{cal/mol}}{\rm{.}}\) \({\rm{\Delta }}{{\rm{H}}_{\rm{f}}}\) for \(\mathrm{\mathrm{H}_{2} \mathrm{O}}\) and \(\mathrm{\mathrm{CO}_{2}}\) are \({\rm{ - 68}}\)\(\mathrm{\mathrm{kcal} / \mathrm{mol}}\) and \({\rm{ - 94}}\,{\rm{k}}\,{\rm{cal/mol}}\), respectively. Then, \(\mathrm{\Delta H}\) for the isomerisation reaction \(\mathrm{\mathrm{CH}_{3} \mathrm{OCH}_{3(\mathrm{~g})}}\) is
369494
Given,
\({{\rm{N}}{{\rm{H}}_3}(\;{\rm{g}}) + 3{\rm{C}}{{\rm{l}}_2}(\;{\rm{g}})}\)\( \rightleftharpoons \)\({{\rm{NC}}{{\rm{l}}_3}(\;{\rm{g}}) + 3{\rm{HCl}}({\rm{g}});}\)
\({ - {\rm{\Delta }}{{\rm{H}}_1}}\)
\({{{\rm{N}}_2}({\rm{g}}) + 3{{\rm{H}}_2}({\rm{g}})}\)\( \rightleftharpoons \)\({2{\rm{N}}{{\rm{H}}_3}({\rm{g}});}\)
\({ - {\rm{\Delta }}{{\rm{H}}_2}}\)
\({{\rm{H}}_{\rm{2}}}{\rm{(g)}} + {\rm{C}}{{\rm{l}}_{\rm{2}}}{\rm{(g)}}\)\( \rightleftharpoons \)\(2{\rm{HCl(g)}};\)
\({\rm{\Delta }}{{\rm{H}}_{\rm{3}}}\)
The heat of formation of \(\mathrm{NCl}_{3}(\mathrm{~g})\)
in terms of \(\Delta \mathrm{H}_{1}, \Delta \mathrm{H}_{2}\) and \(\Delta \mathrm{H}_{3}\) is
369495
In the reaction,
\({\rm{S + }}\frac{{\rm{3}}}{{\rm{2}}}{{\rm{O}}_{\rm{2}}} \to {\rm{S}}{{\rm{O}}_{\rm{3}}}{\rm{ + 2xkJ}}\) and \({\rm{S}}{{\rm{O}}_{\rm{2}}}{\rm{ + }}\frac{{\rm{1}}}{{\rm{2}}}{{\rm{O}}_{\rm{2}}} \to {\rm{S}}{{\rm{O}}_{\rm{3}}}{\rm{ + ykJ}}\)
heat of formation of \(\mathrm{\mathrm{SO}_{2}}\) is
369496
Given
\({\rm{C + 2}}\;{\rm{S}} \to {\rm{C}}{{\rm{S}}_{\rm{2}}},{\Delta _{\rm{f}}}{\rm{H}}^\circ {\rm{ = + 117}}.{\rm{0}}\;{\rm{kJ}}\;{\rm{mo}}{{\rm{l}}^{{\rm{ - 1}}}}\)
\({\rm{C + }}{{\rm{O}}_{\rm{2}}} \to {\rm{C}}{{\rm{O}}_{\rm{2}}},{\Delta _{\rm{f}}}{\rm{H}}^\circ {\rm{ = - 393}}.{\rm{0}}\;{\rm{kJ}}\;{\rm{mo}}{{\rm{l}}^{{\rm{ - 1}}}}\)
\(\;{\rm{S + }}{{\rm{O}}_{\rm{2}}} \to {\rm{S}}{{\rm{O}}_{\rm{2}}},{\Delta _{\rm{f}}}{\rm{H}}^\circ {\rm{ = - 297}}.{\rm{0}}\;{\rm{kJ}}\;{\rm{mo}}{{\rm{l}}^{{\rm{ - 1}}}}\)
The heat of combustion of
\(\mathrm{\mathrm{CS}_{2}+3 \mathrm{O}_{2} \rightarrow \mathrm{CO}_{2}+2 \mathrm{SO}_{2}}\) is
369497
A sample of gas changes from \({{\rm{p}}_{\rm{1}}}{\rm{,}}\) \({{\rm{V}}_{\rm{1}}}\) and \({{\rm{T}}_{\rm{1}}}\) to \({{\rm{p}}_{\rm{2}}}{\rm{,}}\) \({{\rm{V}}_{\rm{2}}}\) and \({{\rm{T}}_{\rm{2}}}\) by one path and then back to \({{\rm{p}}_{\rm{1}}}{\rm{,}}\) \({{\rm{V}}_{\rm{1}}}\) and \({{\rm{T}}_{\rm{1}}}\) by another path. How many of the following must be zero for the gas in this cycle?
\(\Delta {\rm{T}},\Delta \,{\rm{p}},\Delta {\rm{V}},\,\,{\rm{q}}{\rm{.}}\,\,{\rm{W}}\) and \(\Delta \,{\rm{E}},\)