313990
Identify the correct match.
Column I
Column II
A
P
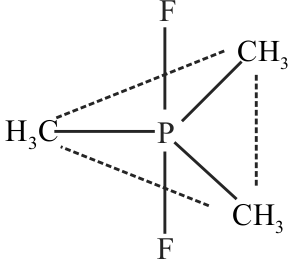
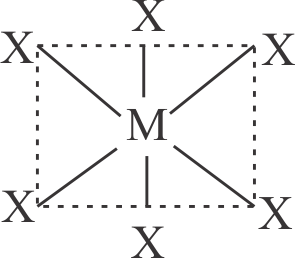
Central atom has
B
Q
Central atom has
C
R
Central atom has
D
S
Central atom has
313990
Identify the correct match.
Column I
Column II
A
P
Central atom has
B
Q
Central atom has
C
R
Central atom has
D
S
Central atom has
313990
Identify the correct match.
Column I
Column II
A
P
Central atom has
B
Q
Central atom has
C
R
Central atom has
D
S
Central atom has
313990
Identify the correct match.
Column I
Column II
A
P
Central atom has
B
Q
Central atom has
C
R
Central atom has
D
S
Central atom has