313996
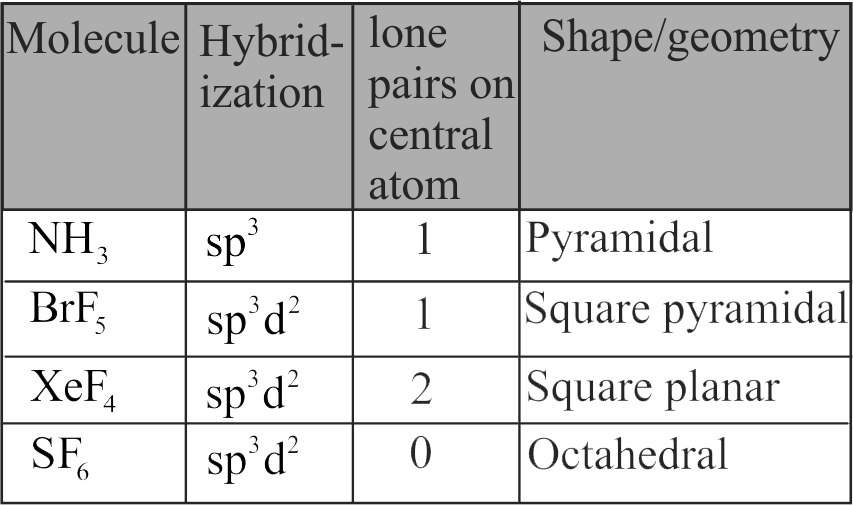
Match Column I (Compound) with Column II (Shape/geometry).
Column I
Column II
A
\({\rm{N}}{{\rm{H}}_{\rm{3}}}\)
P
Trigonal pyramidal
B
\({\rm{Br}}{{\rm{F}}_{\rm{5}}}\)
Q
Square pyramidal
C
\({\rm{Xe}}{{\rm{F}}_{\rm{4}}}\)
R
Octahedral
D
\({\rm{S}}{{\rm{F}}_{\rm{6}}}\)
S
Square pyramidal
313996
Match Column I (Compound) with Column II (Shape/geometry).
Column I
Column II
A
\({\rm{N}}{{\rm{H}}_{\rm{3}}}\)
P
Trigonal pyramidal
B
\({\rm{Br}}{{\rm{F}}_{\rm{5}}}\)
Q
Square pyramidal
C
\({\rm{Xe}}{{\rm{F}}_{\rm{4}}}\)
R
Octahedral
D
\({\rm{S}}{{\rm{F}}_{\rm{6}}}\)
S
Square pyramidal
313996
Match Column I (Compound) with Column II (Shape/geometry).
Column I
Column II
A
\({\rm{N}}{{\rm{H}}_{\rm{3}}}\)
P
Trigonal pyramidal
B
\({\rm{Br}}{{\rm{F}}_{\rm{5}}}\)
Q
Square pyramidal
C
\({\rm{Xe}}{{\rm{F}}_{\rm{4}}}\)
R
Octahedral
D
\({\rm{S}}{{\rm{F}}_{\rm{6}}}\)
S
Square pyramidal
313996
Match Column I (Compound) with Column II (Shape/geometry).
Column I
Column II
A
\({\rm{N}}{{\rm{H}}_{\rm{3}}}\)
P
Trigonal pyramidal
B
\({\rm{Br}}{{\rm{F}}_{\rm{5}}}\)
Q
Square pyramidal
C
\({\rm{Xe}}{{\rm{F}}_{\rm{4}}}\)
R
Octahedral
D
\({\rm{S}}{{\rm{F}}_{\rm{6}}}\)
S
Square pyramidal