313990
Identify the correct match.
Column I
Column II
A
\({\rm{Xe}}{{\rm{F}}_{\rm{2}}}\)
P
Central atom has \({\rm{s}}{{\rm{p}}^{\rm{3}}}\) hybridisation and bent geometry
B
\({\rm{ N}}_3^ - \)
Q
Central atom has \({\rm{s}}{{\rm{p}}^{\rm{3}}}{{\rm{d}}^{\rm{2}}}\) hybridisation and bent octahedral
C
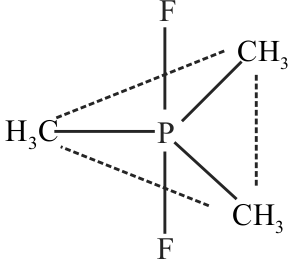
\({\text{PCl}}_{\text{6}}^{\text{ - }}\left( {{\text{PC}}{{\text{l}}_{\text{5}}}{\text{(s) anion}}} \right)\)
R
Central atom has \({\rm{ sp}}\) hybridisation and linear geometry
D
\({\text{ICl}}_{\text{2}}^{\text{ + }}\left( {{{\text{I}}_{\text{2}}}{\text{C}}{{\text{l}}_{\text{6}}}{\text{(l}})\,\,{\text{cation}}} \right)\)
S
Central atom has \({\rm{s}}{{\rm{p}}^{\rm{3}}}{\rm{d}}\) hybridisation and linear geometry
313990
Identify the correct match.
Column I
Column II
A
\({\rm{Xe}}{{\rm{F}}_{\rm{2}}}\)
P
Central atom has \({\rm{s}}{{\rm{p}}^{\rm{3}}}\) hybridisation and bent geometry
B
\({\rm{ N}}_3^ - \)
Q
Central atom has \({\rm{s}}{{\rm{p}}^{\rm{3}}}{{\rm{d}}^{\rm{2}}}\) hybridisation and bent octahedral
C
\({\text{PCl}}_{\text{6}}^{\text{ - }}\left( {{\text{PC}}{{\text{l}}_{\text{5}}}{\text{(s) anion}}} \right)\)
R
Central atom has \({\rm{ sp}}\) hybridisation and linear geometry
D
\({\text{ICl}}_{\text{2}}^{\text{ + }}\left( {{{\text{I}}_{\text{2}}}{\text{C}}{{\text{l}}_{\text{6}}}{\text{(l}})\,\,{\text{cation}}} \right)\)
S
Central atom has \({\rm{s}}{{\rm{p}}^{\rm{3}}}{\rm{d}}\) hybridisation and linear geometry
313990
Identify the correct match.
Column I
Column II
A
\({\rm{Xe}}{{\rm{F}}_{\rm{2}}}\)
P
Central atom has \({\rm{s}}{{\rm{p}}^{\rm{3}}}\) hybridisation and bent geometry
B
\({\rm{ N}}_3^ - \)
Q
Central atom has \({\rm{s}}{{\rm{p}}^{\rm{3}}}{{\rm{d}}^{\rm{2}}}\) hybridisation and bent octahedral
C
\({\text{PCl}}_{\text{6}}^{\text{ - }}\left( {{\text{PC}}{{\text{l}}_{\text{5}}}{\text{(s) anion}}} \right)\)
R
Central atom has \({\rm{ sp}}\) hybridisation and linear geometry
D
\({\text{ICl}}_{\text{2}}^{\text{ + }}\left( {{{\text{I}}_{\text{2}}}{\text{C}}{{\text{l}}_{\text{6}}}{\text{(l}})\,\,{\text{cation}}} \right)\)
S
Central atom has \({\rm{s}}{{\rm{p}}^{\rm{3}}}{\rm{d}}\) hybridisation and linear geometry
313990
Identify the correct match.
Column I
Column II
A
\({\rm{Xe}}{{\rm{F}}_{\rm{2}}}\)
P
Central atom has \({\rm{s}}{{\rm{p}}^{\rm{3}}}\) hybridisation and bent geometry
B
\({\rm{ N}}_3^ - \)
Q
Central atom has \({\rm{s}}{{\rm{p}}^{\rm{3}}}{{\rm{d}}^{\rm{2}}}\) hybridisation and bent octahedral
C
\({\text{PCl}}_{\text{6}}^{\text{ - }}\left( {{\text{PC}}{{\text{l}}_{\text{5}}}{\text{(s) anion}}} \right)\)
R
Central atom has \({\rm{ sp}}\) hybridisation and linear geometry
D
\({\text{ICl}}_{\text{2}}^{\text{ + }}\left( {{{\text{I}}_{\text{2}}}{\text{C}}{{\text{l}}_{\text{6}}}{\text{(l}})\,\,{\text{cation}}} \right)\)
S
Central atom has \({\rm{s}}{{\rm{p}}^{\rm{3}}}{\rm{d}}\) hybridisation and linear geometry