371287
A graph is drawn between coefficient of volume expansion of the gas \(\alpha\) and temperature \(T\) of a gas having \(n = 2\) and \(\gamma=1.5\). Given that \(VT = K\) where \(K\) is a constant. Find the area of the graph between \(|\alpha|\) and \(T\), w.r.to \(T\)- axis from \(\dfrac{T_{\mathrm{o}}}{3}\) to \(T_{\text {o }}\)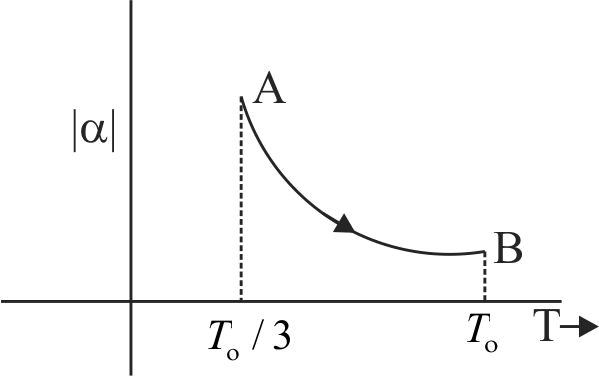
371287
A graph is drawn between coefficient of volume expansion of the gas \(\alpha\) and temperature \(T\) of a gas having \(n = 2\) and \(\gamma=1.5\). Given that \(VT = K\) where \(K\) is a constant. Find the area of the graph between \(|\alpha|\) and \(T\), w.r.to \(T\)- axis from \(\dfrac{T_{\mathrm{o}}}{3}\) to \(T_{\text {o }}\)
371287
A graph is drawn between coefficient of volume expansion of the gas \(\alpha\) and temperature \(T\) of a gas having \(n = 2\) and \(\gamma=1.5\). Given that \(VT = K\) where \(K\) is a constant. Find the area of the graph between \(|\alpha|\) and \(T\), w.r.to \(T\)- axis from \(\dfrac{T_{\mathrm{o}}}{3}\) to \(T_{\text {o }}\)
371287
A graph is drawn between coefficient of volume expansion of the gas \(\alpha\) and temperature \(T\) of a gas having \(n = 2\) and \(\gamma=1.5\). Given that \(VT = K\) where \(K\) is a constant. Find the area of the graph between \(|\alpha|\) and \(T\), w.r.to \(T\)- axis from \(\dfrac{T_{\mathrm{o}}}{3}\) to \(T_{\text {o }}\)