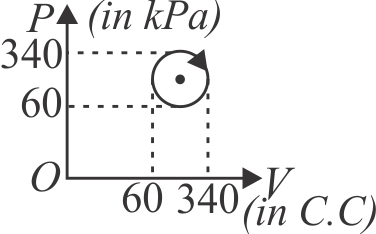
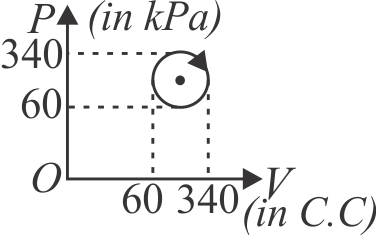
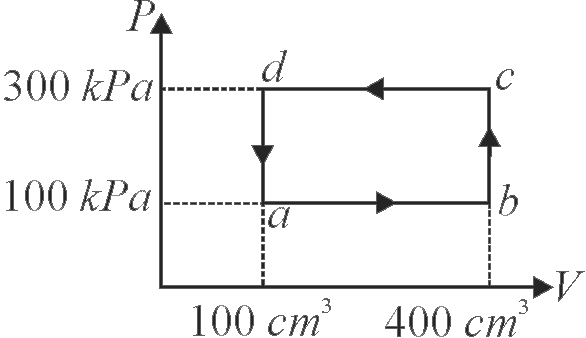
371280 An ideal gas is taken through a cyclic thermodynamical process through four steps. The amounts of heat involved in these steps are:\({Q_1} = 5960\,J,{Q_2} = - 5585\,J,{Q_3} = - 2980\,J\), \({Q_4} = 3645\,J\) respectively. The corresponding works involved are \({W_1} = 2200\,J,{W_2} = - 825\,J,{W_3} = - 1100\,J\) and \(W_{4}\) respectively. The value of \(W_{4}\) is :
371282
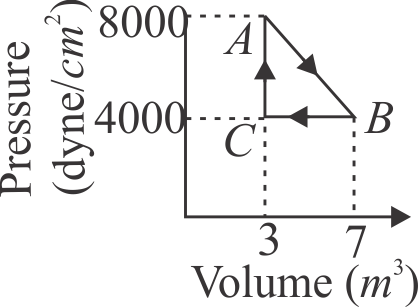
A thermodynamic system is taken from an original state \({A}\) to an intermediate state \({B}\) by a linear process as shown in the figure. It's volume is then reduced to the original value from to by an isobaric process. The total work done by the gas from \({A}\) to \({B}\) and \({B}\) to \({C}\) would be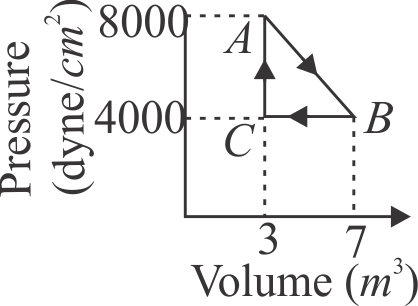
371280 An ideal gas is taken through a cyclic thermodynamical process through four steps. The amounts of heat involved in these steps are:\({Q_1} = 5960\,J,{Q_2} = - 5585\,J,{Q_3} = - 2980\,J\), \({Q_4} = 3645\,J\) respectively. The corresponding works involved are \({W_1} = 2200\,J,{W_2} = - 825\,J,{W_3} = - 1100\,J\) and \(W_{4}\) respectively. The value of \(W_{4}\) is :
371282
A thermodynamic system is taken from an original state \({A}\) to an intermediate state \({B}\) by a linear process as shown in the figure. It's volume is then reduced to the original value from to by an isobaric process. The total work done by the gas from \({A}\) to \({B}\) and \({B}\) to \({C}\) would be
371280 An ideal gas is taken through a cyclic thermodynamical process through four steps. The amounts of heat involved in these steps are:\({Q_1} = 5960\,J,{Q_2} = - 5585\,J,{Q_3} = - 2980\,J\), \({Q_4} = 3645\,J\) respectively. The corresponding works involved are \({W_1} = 2200\,J,{W_2} = - 825\,J,{W_3} = - 1100\,J\) and \(W_{4}\) respectively. The value of \(W_{4}\) is :
371282
A thermodynamic system is taken from an original state \({A}\) to an intermediate state \({B}\) by a linear process as shown in the figure. It's volume is then reduced to the original value from to by an isobaric process. The total work done by the gas from \({A}\) to \({B}\) and \({B}\) to \({C}\) would be
371280 An ideal gas is taken through a cyclic thermodynamical process through four steps. The amounts of heat involved in these steps are:\({Q_1} = 5960\,J,{Q_2} = - 5585\,J,{Q_3} = - 2980\,J\), \({Q_4} = 3645\,J\) respectively. The corresponding works involved are \({W_1} = 2200\,J,{W_2} = - 825\,J,{W_3} = - 1100\,J\) and \(W_{4}\) respectively. The value of \(W_{4}\) is :
371282
A thermodynamic system is taken from an original state \({A}\) to an intermediate state \({B}\) by a linear process as shown in the figure. It's volume is then reduced to the original value from to by an isobaric process. The total work done by the gas from \({A}\) to \({B}\) and \({B}\) to \({C}\) would be