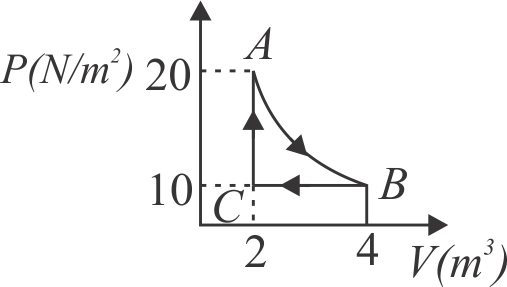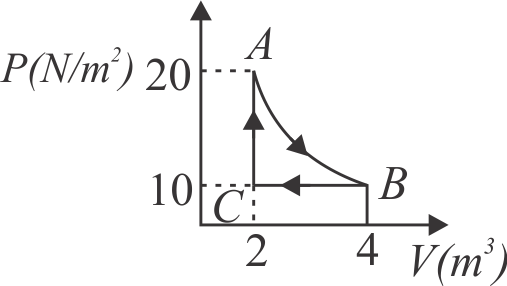371267
Amongst the two paths that may be taken by a gas to go from a state \(A\) to a state \(C\), in process \(AB\) (as shown in figure), \(360\;J\) of heat is added to the system. In process \(BC,140\;J\) of heat is added to the system. The heat absorbed by the system in the process \(AC\) will be
371269
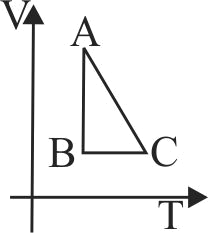
Two moles of an ideal monoatomic gas are taken through a cycle \(A B C A\) as shown in the \(P T\) diagram in figure. During the process \(A B\), pressure and temperature of the gas vary such that \(P T=\) constant. If \(T_{0}=300 {~K}\), and the heat released by the gas in the process \(A B\) (in \(kJ\)).
\((R = 8.3\,J/mo{l^{ - K}})\)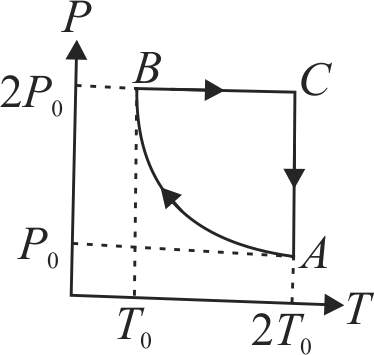
371270
A real gas within a closed chamber at \({27^{\circ} {C}, P\left({~N} / {m}^{2}\right)}\) undergoes the cyclic process as shown in figure. The gas obeys \({P V^{3}=R T}\) equation for the path \({A}\) to \({B}\). The net work done in the complete cycle is
\(({\text{assuming }}\,R = 8\,J/mol\,K)\)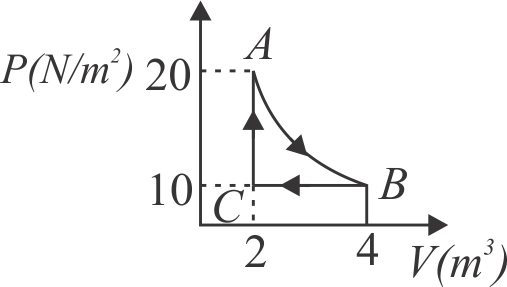
371267
Amongst the two paths that may be taken by a gas to go from a state \(A\) to a state \(C\), in process \(AB\) (as shown in figure), \(360\;J\) of heat is added to the system. In process \(BC,140\;J\) of heat is added to the system. The heat absorbed by the system in the process \(AC\) will be
371269
Two moles of an ideal monoatomic gas are taken through a cycle \(A B C A\) as shown in the \(P T\) diagram in figure. During the process \(A B\), pressure and temperature of the gas vary such that \(P T=\) constant. If \(T_{0}=300 {~K}\), and the heat released by the gas in the process \(A B\) (in \(kJ\)).
\((R = 8.3\,J/mo{l^{ - K}})\)
371270
A real gas within a closed chamber at \({27^{\circ} {C}, P\left({~N} / {m}^{2}\right)}\) undergoes the cyclic process as shown in figure. The gas obeys \({P V^{3}=R T}\) equation for the path \({A}\) to \({B}\). The net work done in the complete cycle is
\(({\text{assuming }}\,R = 8\,J/mol\,K)\)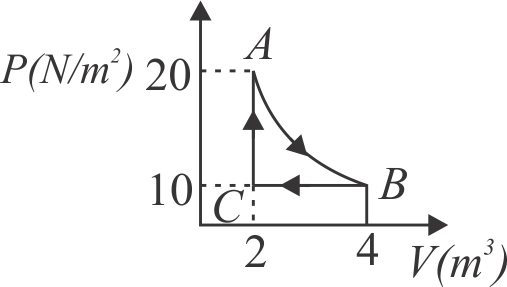
371267
Amongst the two paths that may be taken by a gas to go from a state \(A\) to a state \(C\), in process \(AB\) (as shown in figure), \(360\;J\) of heat is added to the system. In process \(BC,140\;J\) of heat is added to the system. The heat absorbed by the system in the process \(AC\) will be
371269
Two moles of an ideal monoatomic gas are taken through a cycle \(A B C A\) as shown in the \(P T\) diagram in figure. During the process \(A B\), pressure and temperature of the gas vary such that \(P T=\) constant. If \(T_{0}=300 {~K}\), and the heat released by the gas in the process \(A B\) (in \(kJ\)).
\((R = 8.3\,J/mo{l^{ - K}})\)
371270
A real gas within a closed chamber at \({27^{\circ} {C}, P\left({~N} / {m}^{2}\right)}\) undergoes the cyclic process as shown in figure. The gas obeys \({P V^{3}=R T}\) equation for the path \({A}\) to \({B}\). The net work done in the complete cycle is
\(({\text{assuming }}\,R = 8\,J/mol\,K)\)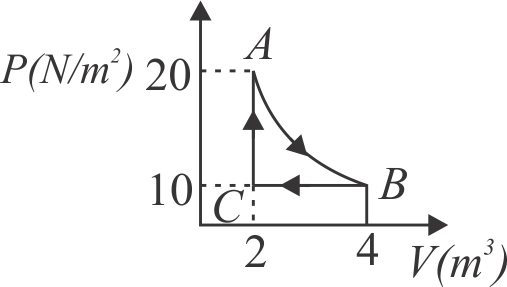
371267
Amongst the two paths that may be taken by a gas to go from a state \(A\) to a state \(C\), in process \(AB\) (as shown in figure), \(360\;J\) of heat is added to the system. In process \(BC,140\;J\) of heat is added to the system. The heat absorbed by the system in the process \(AC\) will be
371269
Two moles of an ideal monoatomic gas are taken through a cycle \(A B C A\) as shown in the \(P T\) diagram in figure. During the process \(A B\), pressure and temperature of the gas vary such that \(P T=\) constant. If \(T_{0}=300 {~K}\), and the heat released by the gas in the process \(A B\) (in \(kJ\)).
\((R = 8.3\,J/mo{l^{ - K}})\)
371270
A real gas within a closed chamber at \({27^{\circ} {C}, P\left({~N} / {m}^{2}\right)}\) undergoes the cyclic process as shown in figure. The gas obeys \({P V^{3}=R T}\) equation for the path \({A}\) to \({B}\). The net work done in the complete cycle is
\(({\text{assuming }}\,R = 8\,J/mol\,K)\)