371274
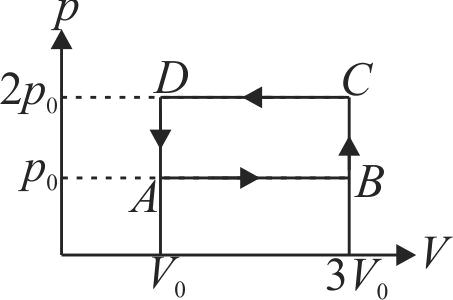
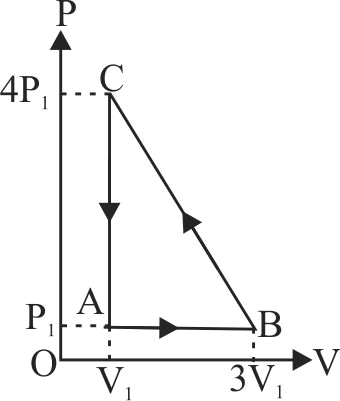
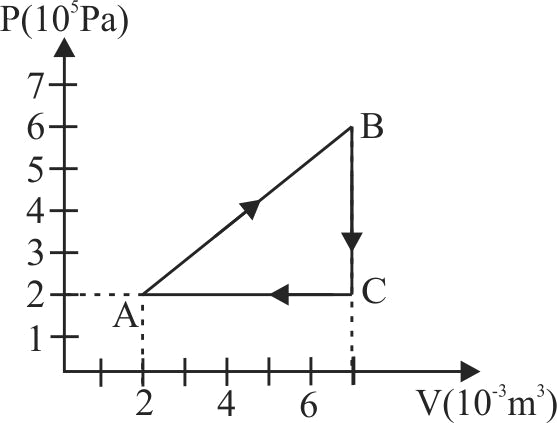
The \(P-V\) diagram of a system undergoing thermodynamic transformation is shown in figure. The work done by the system in going from \(A \rightarrow B \rightarrow C\) is \(30\;J\), and \(40\;J\) heat is given to the system. The change in internal energy between \(A\) and \(C\) is
371274
The \(P-V\) diagram of a system undergoing thermodynamic transformation is shown in figure. The work done by the system in going from \(A \rightarrow B \rightarrow C\) is \(30\;J\), and \(40\;J\) heat is given to the system. The change in internal energy between \(A\) and \(C\) is
371274
The \(P-V\) diagram of a system undergoing thermodynamic transformation is shown in figure. The work done by the system in going from \(A \rightarrow B \rightarrow C\) is \(30\;J\), and \(40\;J\) heat is given to the system. The change in internal energy between \(A\) and \(C\) is
371274
The \(P-V\) diagram of a system undergoing thermodynamic transformation is shown in figure. The work done by the system in going from \(A \rightarrow B \rightarrow C\) is \(30\;J\), and \(40\;J\) heat is given to the system. The change in internal energy between \(A\) and \(C\) is
371274
The \(P-V\) diagram of a system undergoing thermodynamic transformation is shown in figure. The work done by the system in going from \(A \rightarrow B \rightarrow C\) is \(30\;J\), and \(40\;J\) heat is given to the system. The change in internal energy between \(A\) and \(C\) is