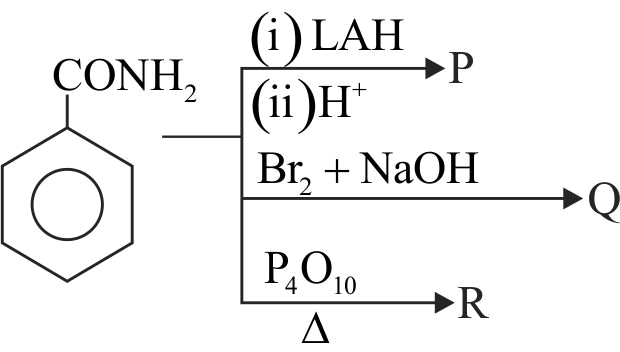
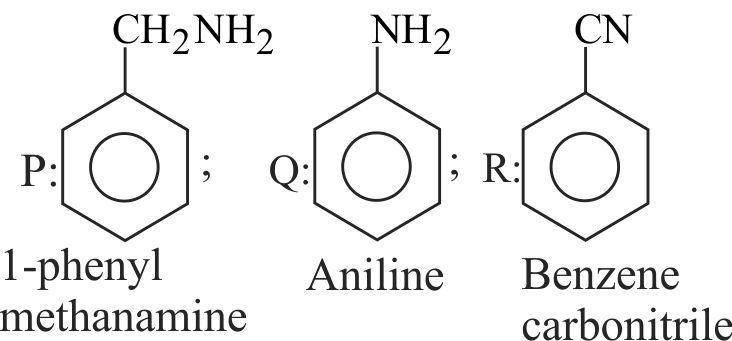
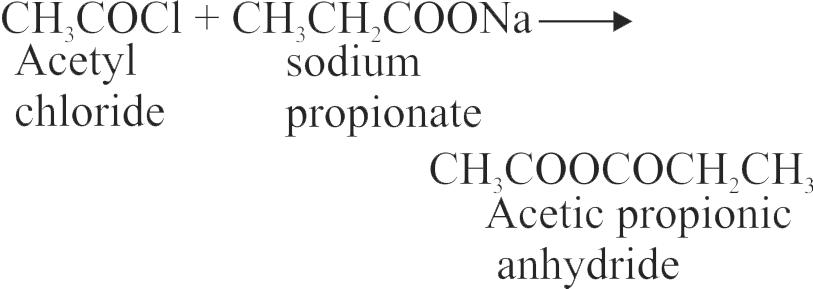
323412
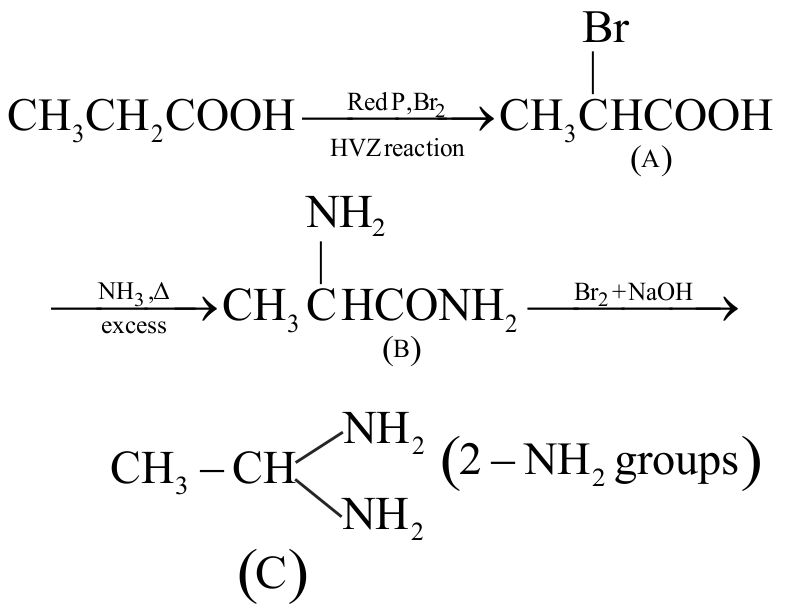
\({\rm{C}}{{\rm{H}}_{\rm{3}}}{\rm{C}}{{\rm{H}}_{\rm{2}}}{\rm{COOH}}\) \(\xrightarrow{{{\text{RedP,B}}{{\text{r}}_{\text{2}}}}}{\text{A}}\) \(\xrightarrow[{{\text{excess}}}]{{{\text{N}}{{\text{H}}_{\text{3}}},\Delta }}{\text{B}}\) \(\xrightarrow{{{\text{B}}{{\text{r}}_{\text{2}}}{\text{ + NaOH}}}}{\text{C}}\)
The number of \(-\mathrm{NH}_{2}\) groups in compound (C) is
323412
\({\rm{C}}{{\rm{H}}_{\rm{3}}}{\rm{C}}{{\rm{H}}_{\rm{2}}}{\rm{COOH}}\) \(\xrightarrow{{{\text{RedP,B}}{{\text{r}}_{\text{2}}}}}{\text{A}}\) \(\xrightarrow[{{\text{excess}}}]{{{\text{N}}{{\text{H}}_{\text{3}}},\Delta }}{\text{B}}\) \(\xrightarrow{{{\text{B}}{{\text{r}}_{\text{2}}}{\text{ + NaOH}}}}{\text{C}}\)
The number of \(-\mathrm{NH}_{2}\) groups in compound (C) is
323412
\({\rm{C}}{{\rm{H}}_{\rm{3}}}{\rm{C}}{{\rm{H}}_{\rm{2}}}{\rm{COOH}}\) \(\xrightarrow{{{\text{RedP,B}}{{\text{r}}_{\text{2}}}}}{\text{A}}\) \(\xrightarrow[{{\text{excess}}}]{{{\text{N}}{{\text{H}}_{\text{3}}},\Delta }}{\text{B}}\) \(\xrightarrow{{{\text{B}}{{\text{r}}_{\text{2}}}{\text{ + NaOH}}}}{\text{C}}\)
The number of \(-\mathrm{NH}_{2}\) groups in compound (C) is
323412
\({\rm{C}}{{\rm{H}}_{\rm{3}}}{\rm{C}}{{\rm{H}}_{\rm{2}}}{\rm{COOH}}\) \(\xrightarrow{{{\text{RedP,B}}{{\text{r}}_{\text{2}}}}}{\text{A}}\) \(\xrightarrow[{{\text{excess}}}]{{{\text{N}}{{\text{H}}_{\text{3}}},\Delta }}{\text{B}}\) \(\xrightarrow{{{\text{B}}{{\text{r}}_{\text{2}}}{\text{ + NaOH}}}}{\text{C}}\)
The number of \(-\mathrm{NH}_{2}\) groups in compound (C) is