Explanation:
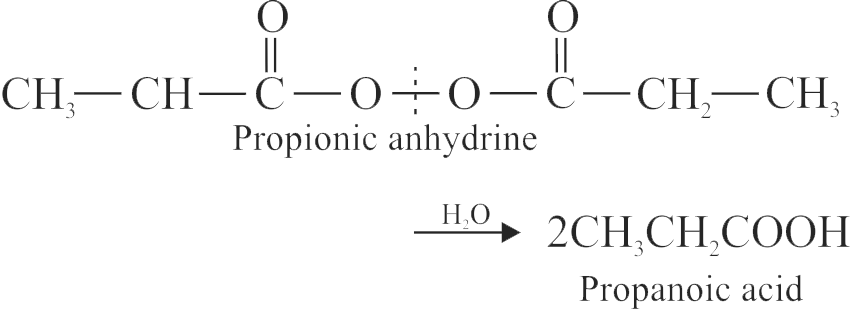
\({\mathrm{1 \mathrm{~mol}(130 \, \mathrm{gm})=2 \mathrm{~mol}}}\)
\(\,\,\,\,\,\,\,\,\,\,\,\,\)\(1 \mathrm{gm}=\dfrac{2}{130} \mathrm{~mol}\)
No. of equivalents of acid = number of equivalents of base.
\({\mathrm{\dfrac{2}{130} \times 1000 \mathrm{~m}}}\) equiv \({\mathrm{=0.5 \times \mathrm{V}}}\)
\(\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\)\(V=44.4 \mathrm{ml}\)