323407
The product ' \(\mathrm{C}\) ' in the following reaction is
\({\text{RCOOH}}\xrightarrow{{{\text{N}}{{\text{H}}_{\text{3}}}}}{\text{(A)}}\xrightarrow{{{\text{heat}}}}{\text{(B)}}\xrightarrow{{{{\text{P}}_{\text{2}}}{{\text{O}}_{\text{5}}}{\text{, heat}}}}{\text{(C)}}\)
323409
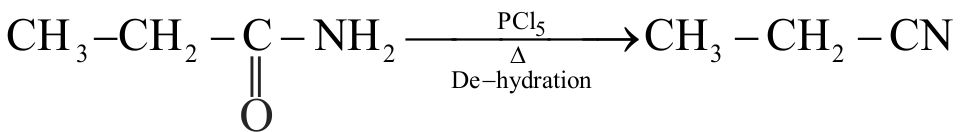
In a set of reaction, propionic acid yielded the compound (D) :
\({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{COOH}}\xrightarrow{{{\text{SOC}}{{\text{l}}_{\text{2}}}}}{\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{COCl(B)}}\)
\(\xrightarrow{{{\text{N}}{{\text{H}}_{\text{3}}}}}{\text{(C)}}\xrightarrow{{{\text{KOH/B}}{{\text{r}}_{\text{2}}}}}{\text{(D) }}\)
The structure of compound (D) will be
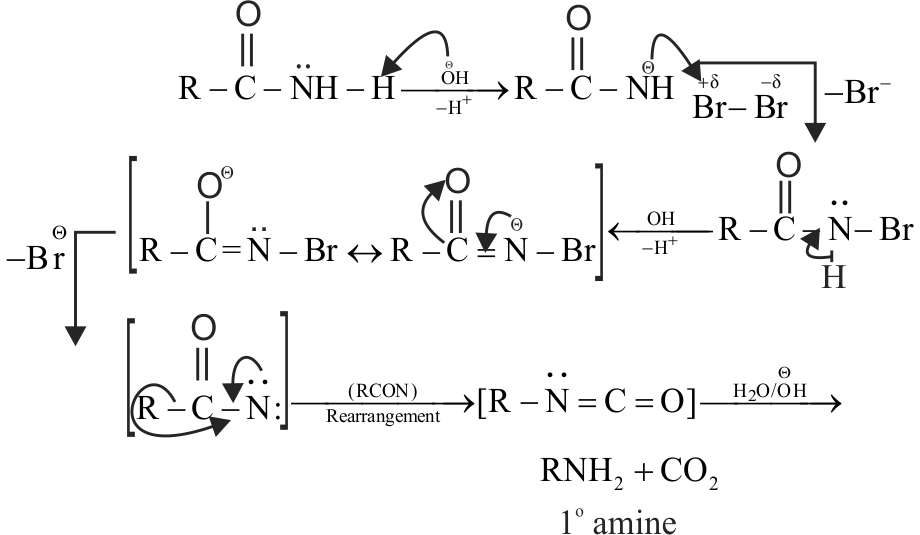
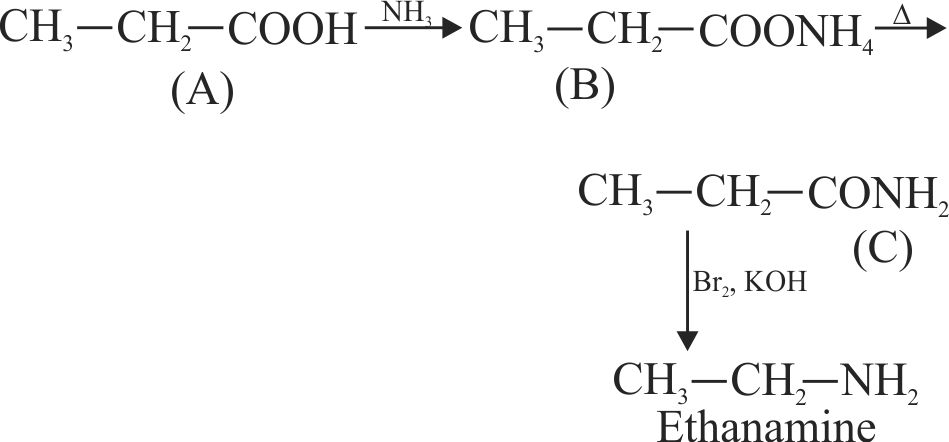
323410 An organic compound ' \({\rm{A}}\) ' on treatment with \(\mathrm{NH}_{3}\) gives ' \(\mathrm{B}\) ' which on heating gives ' \(\mathrm{C}\) ' when treated with \(\mathrm{Br}_{2}\) in presence of \(\mathrm{KOH}\) produces ethylamine. Compound ' \(\mathrm{A}\) ' is
323407
The product ' \(\mathrm{C}\) ' in the following reaction is
\({\text{RCOOH}}\xrightarrow{{{\text{N}}{{\text{H}}_{\text{3}}}}}{\text{(A)}}\xrightarrow{{{\text{heat}}}}{\text{(B)}}\xrightarrow{{{{\text{P}}_{\text{2}}}{{\text{O}}_{\text{5}}}{\text{, heat}}}}{\text{(C)}}\)
323409
In a set of reaction, propionic acid yielded the compound (D) :
\({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{COOH}}\xrightarrow{{{\text{SOC}}{{\text{l}}_{\text{2}}}}}{\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{COCl(B)}}\)
\(\xrightarrow{{{\text{N}}{{\text{H}}_{\text{3}}}}}{\text{(C)}}\xrightarrow{{{\text{KOH/B}}{{\text{r}}_{\text{2}}}}}{\text{(D) }}\)
The structure of compound (D) will be
323410 An organic compound ' \({\rm{A}}\) ' on treatment with \(\mathrm{NH}_{3}\) gives ' \(\mathrm{B}\) ' which on heating gives ' \(\mathrm{C}\) ' when treated with \(\mathrm{Br}_{2}\) in presence of \(\mathrm{KOH}\) produces ethylamine. Compound ' \(\mathrm{A}\) ' is
323407
The product ' \(\mathrm{C}\) ' in the following reaction is
\({\text{RCOOH}}\xrightarrow{{{\text{N}}{{\text{H}}_{\text{3}}}}}{\text{(A)}}\xrightarrow{{{\text{heat}}}}{\text{(B)}}\xrightarrow{{{{\text{P}}_{\text{2}}}{{\text{O}}_{\text{5}}}{\text{, heat}}}}{\text{(C)}}\)
323409
In a set of reaction, propionic acid yielded the compound (D) :
\({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{COOH}}\xrightarrow{{{\text{SOC}}{{\text{l}}_{\text{2}}}}}{\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{COCl(B)}}\)
\(\xrightarrow{{{\text{N}}{{\text{H}}_{\text{3}}}}}{\text{(C)}}\xrightarrow{{{\text{KOH/B}}{{\text{r}}_{\text{2}}}}}{\text{(D) }}\)
The structure of compound (D) will be
323410 An organic compound ' \({\rm{A}}\) ' on treatment with \(\mathrm{NH}_{3}\) gives ' \(\mathrm{B}\) ' which on heating gives ' \(\mathrm{C}\) ' when treated with \(\mathrm{Br}_{2}\) in presence of \(\mathrm{KOH}\) produces ethylamine. Compound ' \(\mathrm{A}\) ' is
323407
The product ' \(\mathrm{C}\) ' in the following reaction is
\({\text{RCOOH}}\xrightarrow{{{\text{N}}{{\text{H}}_{\text{3}}}}}{\text{(A)}}\xrightarrow{{{\text{heat}}}}{\text{(B)}}\xrightarrow{{{{\text{P}}_{\text{2}}}{{\text{O}}_{\text{5}}}{\text{, heat}}}}{\text{(C)}}\)
323409
In a set of reaction, propionic acid yielded the compound (D) :
\({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{COOH}}\xrightarrow{{{\text{SOC}}{{\text{l}}_{\text{2}}}}}{\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{COCl(B)}}\)
\(\xrightarrow{{{\text{N}}{{\text{H}}_{\text{3}}}}}{\text{(C)}}\xrightarrow{{{\text{KOH/B}}{{\text{r}}_{\text{2}}}}}{\text{(D) }}\)
The structure of compound (D) will be
323410 An organic compound ' \({\rm{A}}\) ' on treatment with \(\mathrm{NH}_{3}\) gives ' \(\mathrm{B}\) ' which on heating gives ' \(\mathrm{C}\) ' when treated with \(\mathrm{Br}_{2}\) in presence of \(\mathrm{KOH}\) produces ethylamine. Compound ' \(\mathrm{A}\) ' is
323407
The product ' \(\mathrm{C}\) ' in the following reaction is
\({\text{RCOOH}}\xrightarrow{{{\text{N}}{{\text{H}}_{\text{3}}}}}{\text{(A)}}\xrightarrow{{{\text{heat}}}}{\text{(B)}}\xrightarrow{{{{\text{P}}_{\text{2}}}{{\text{O}}_{\text{5}}}{\text{, heat}}}}{\text{(C)}}\)
323409
In a set of reaction, propionic acid yielded the compound (D) :
\({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{COOH}}\xrightarrow{{{\text{SOC}}{{\text{l}}_{\text{2}}}}}{\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{COCl(B)}}\)
\(\xrightarrow{{{\text{N}}{{\text{H}}_{\text{3}}}}}{\text{(C)}}\xrightarrow{{{\text{KOH/B}}{{\text{r}}_{\text{2}}}}}{\text{(D) }}\)
The structure of compound (D) will be
323410 An organic compound ' \({\rm{A}}\) ' on treatment with \(\mathrm{NH}_{3}\) gives ' \(\mathrm{B}\) ' which on heating gives ' \(\mathrm{C}\) ' when treated with \(\mathrm{Br}_{2}\) in presence of \(\mathrm{KOH}\) produces ethylamine. Compound ' \(\mathrm{A}\) ' is