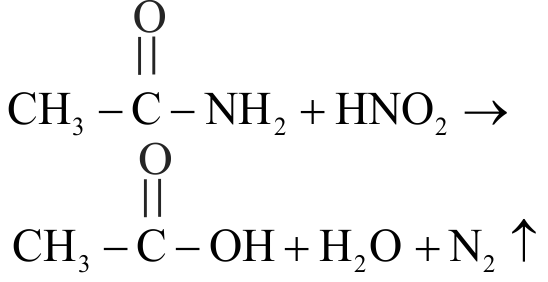CHXII12:ALDEHYDES KETONES AND CARBOXYLIC ACIDS
323405
The main product obtained in the reaction of acetamide and \(\mathrm{HNO}_{2}\) is
1 \(\mathrm{CH}_{3} \mathrm{CN}\)
2 \(\mathrm{CH}_{3} \mathrm{NC}\)
3 \(\mathrm{CH}_{3} \mathrm{NH}_{2}\)
4 \(\mathrm{CH}_{3} \mathrm{COOH}\)
Explanation: