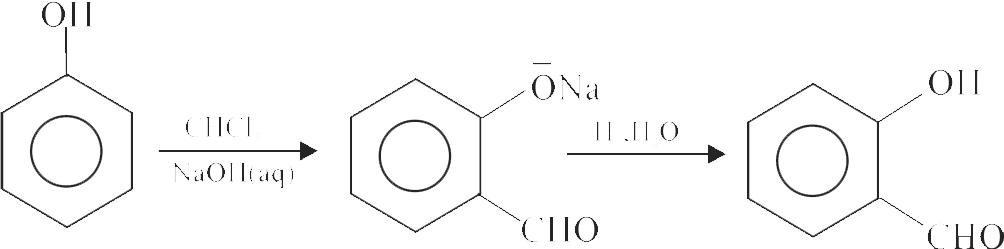
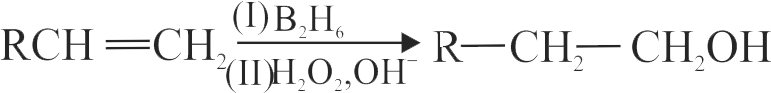
323011
Consider the following statements:
I. Solubility decreases with increase in size of alkyl/aryl groups.
II. Alcohols and phenols react with active metals to give alkoxides or phenoxides.
III. Alcohols act as Bronsted acid in the presence of electrophiles.
Identify the correct statement (s)
323011
Consider the following statements:
I. Solubility decreases with increase in size of alkyl/aryl groups.
II. Alcohols and phenols react with active metals to give alkoxides or phenoxides.
III. Alcohols act as Bronsted acid in the presence of electrophiles.
Identify the correct statement (s)
323011
Consider the following statements:
I. Solubility decreases with increase in size of alkyl/aryl groups.
II. Alcohols and phenols react with active metals to give alkoxides or phenoxides.
III. Alcohols act as Bronsted acid in the presence of electrophiles.
Identify the correct statement (s)
323011
Consider the following statements:
I. Solubility decreases with increase in size of alkyl/aryl groups.
II. Alcohols and phenols react with active metals to give alkoxides or phenoxides.
III. Alcohols act as Bronsted acid in the presence of electrophiles.
Identify the correct statement (s)
323011
Consider the following statements:
I. Solubility decreases with increase in size of alkyl/aryl groups.
II. Alcohols and phenols react with active metals to give alkoxides or phenoxides.
III. Alcohols act as Bronsted acid in the presence of electrophiles.
Identify the correct statement (s)