323016
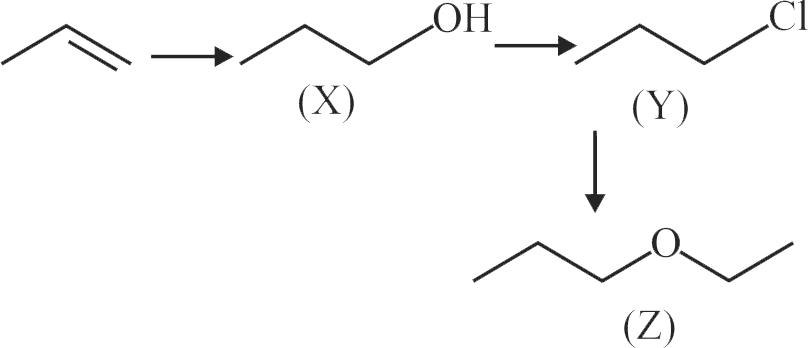
In the following sequence of reactions, the compound \(\mathrm{Z}\) is
\[\begin{gathered}
{\text{C}}{{\text{H}}_{\text{3}}}{\text{CH = C}}{{\text{H}}_{\text{2}}}\xrightarrow[{{\text{ (ii) }}{{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}\,{\text{/}}\,{\text{OH}}}]{{{\text{(i)}}\,\,{\text{B}}{{\text{H}}_{\text{3}}}}}{\text{X}}\xrightarrow[{{\text{ - POC}}{{\text{l}}_{\text{3}}}{\text{ - HCl}}}]{{{\text{PC}}{{\text{l}}_{\text{5}}}}} \hfill \\
\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,{\text{Y}}\xrightarrow{{{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{{\text{O}}^{{\text{ - }}}}{\text{N}}{{\text{a}}^{{\text{ + }}}}}}{\text{Z}}{\text{.}} \hfill \\
\end{gathered} \]
323017
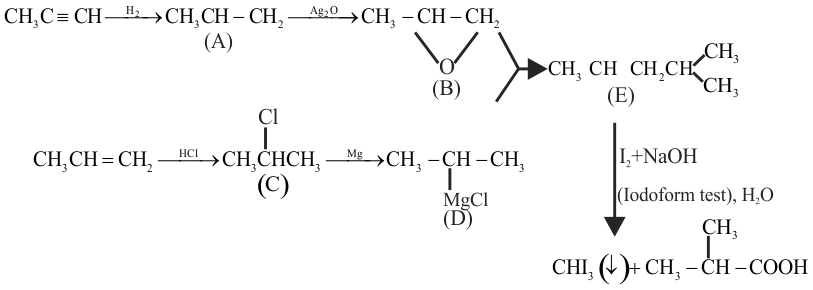
\({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}} \equiv {\text{CH}}\xrightarrow[{{\text{of}}\,{{\text{H}}_{\text{2}}}}]{{{\text{one}}\,{\text{mole}}}}{\text{(A)}}\xrightarrow{{{\text{Dry}}\,{\text{A}}{{\text{g}}_{\text{2}}}{\text{O}}}}{\text{(B)}}\)
\({\text{C}}{{\text{H}}_{\text{3}}}{\text{CH = C}}{{\text{H}}_{\text{2}}}\xrightarrow{{{\text{HCl}}}}{\text{(C)}}\xrightarrow[{{\text{Ether}}}]{{{\text{Mg}}}}{\text{(D) (B) + (D)}}\)
\(\xrightarrow{{{{\text{H}}_{\text{2}}}{\text{O}}}}{\text{(E)}}\xrightarrow[{{{\text{N}}_{\text{2}}}{\text{ + OH}}}]{{{{\text{I}}^{{\text{ + }}}}}}{\text{CH}}{{\text{I}}_{\text{3}}}{\text{(}} \downarrow {\text{) + (F)}}\xrightarrow{{{{\text{H}}_{\text{2}}}{\text{O}}}}{\text{(G)}}\)
The functional group present in compound ' G ' is
323019 Compound 'A' reacts with Na metal to give '\({\text{B}}\) '. ' \({\text{A}}\) ' also reacts with \(\mathrm{PCl}_{5}\) to give ' \({\text{C}}\) '. ' \({\text{B}}\) ' and ' \({\text{C}}\)' reacts with each other to give dimethyl ether. Then ' \({\text{A}}\) ', ' \({\text{B}}\) ' and ' \({\text{C}}\) ' respectively are:
323016
In the following sequence of reactions, the compound \(\mathrm{Z}\) is
\[\begin{gathered}
{\text{C}}{{\text{H}}_{\text{3}}}{\text{CH = C}}{{\text{H}}_{\text{2}}}\xrightarrow[{{\text{ (ii) }}{{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}\,{\text{/}}\,{\text{OH}}}]{{{\text{(i)}}\,\,{\text{B}}{{\text{H}}_{\text{3}}}}}{\text{X}}\xrightarrow[{{\text{ - POC}}{{\text{l}}_{\text{3}}}{\text{ - HCl}}}]{{{\text{PC}}{{\text{l}}_{\text{5}}}}} \hfill \\
\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,{\text{Y}}\xrightarrow{{{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{{\text{O}}^{{\text{ - }}}}{\text{N}}{{\text{a}}^{{\text{ + }}}}}}{\text{Z}}{\text{.}} \hfill \\
\end{gathered} \]
323017
\({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}} \equiv {\text{CH}}\xrightarrow[{{\text{of}}\,{{\text{H}}_{\text{2}}}}]{{{\text{one}}\,{\text{mole}}}}{\text{(A)}}\xrightarrow{{{\text{Dry}}\,{\text{A}}{{\text{g}}_{\text{2}}}{\text{O}}}}{\text{(B)}}\)
\({\text{C}}{{\text{H}}_{\text{3}}}{\text{CH = C}}{{\text{H}}_{\text{2}}}\xrightarrow{{{\text{HCl}}}}{\text{(C)}}\xrightarrow[{{\text{Ether}}}]{{{\text{Mg}}}}{\text{(D) (B) + (D)}}\)
\(\xrightarrow{{{{\text{H}}_{\text{2}}}{\text{O}}}}{\text{(E)}}\xrightarrow[{{{\text{N}}_{\text{2}}}{\text{ + OH}}}]{{{{\text{I}}^{{\text{ + }}}}}}{\text{CH}}{{\text{I}}_{\text{3}}}{\text{(}} \downarrow {\text{) + (F)}}\xrightarrow{{{{\text{H}}_{\text{2}}}{\text{O}}}}{\text{(G)}}\)
The functional group present in compound ' G ' is
323019 Compound 'A' reacts with Na metal to give '\({\text{B}}\) '. ' \({\text{A}}\) ' also reacts with \(\mathrm{PCl}_{5}\) to give ' \({\text{C}}\) '. ' \({\text{B}}\) ' and ' \({\text{C}}\)' reacts with each other to give dimethyl ether. Then ' \({\text{A}}\) ', ' \({\text{B}}\) ' and ' \({\text{C}}\) ' respectively are:
323016
In the following sequence of reactions, the compound \(\mathrm{Z}\) is
\[\begin{gathered}
{\text{C}}{{\text{H}}_{\text{3}}}{\text{CH = C}}{{\text{H}}_{\text{2}}}\xrightarrow[{{\text{ (ii) }}{{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}\,{\text{/}}\,{\text{OH}}}]{{{\text{(i)}}\,\,{\text{B}}{{\text{H}}_{\text{3}}}}}{\text{X}}\xrightarrow[{{\text{ - POC}}{{\text{l}}_{\text{3}}}{\text{ - HCl}}}]{{{\text{PC}}{{\text{l}}_{\text{5}}}}} \hfill \\
\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,{\text{Y}}\xrightarrow{{{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{{\text{O}}^{{\text{ - }}}}{\text{N}}{{\text{a}}^{{\text{ + }}}}}}{\text{Z}}{\text{.}} \hfill \\
\end{gathered} \]
323017
\({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}} \equiv {\text{CH}}\xrightarrow[{{\text{of}}\,{{\text{H}}_{\text{2}}}}]{{{\text{one}}\,{\text{mole}}}}{\text{(A)}}\xrightarrow{{{\text{Dry}}\,{\text{A}}{{\text{g}}_{\text{2}}}{\text{O}}}}{\text{(B)}}\)
\({\text{C}}{{\text{H}}_{\text{3}}}{\text{CH = C}}{{\text{H}}_{\text{2}}}\xrightarrow{{{\text{HCl}}}}{\text{(C)}}\xrightarrow[{{\text{Ether}}}]{{{\text{Mg}}}}{\text{(D) (B) + (D)}}\)
\(\xrightarrow{{{{\text{H}}_{\text{2}}}{\text{O}}}}{\text{(E)}}\xrightarrow[{{{\text{N}}_{\text{2}}}{\text{ + OH}}}]{{{{\text{I}}^{{\text{ + }}}}}}{\text{CH}}{{\text{I}}_{\text{3}}}{\text{(}} \downarrow {\text{) + (F)}}\xrightarrow{{{{\text{H}}_{\text{2}}}{\text{O}}}}{\text{(G)}}\)
The functional group present in compound ' G ' is
323019 Compound 'A' reacts with Na metal to give '\({\text{B}}\) '. ' \({\text{A}}\) ' also reacts with \(\mathrm{PCl}_{5}\) to give ' \({\text{C}}\) '. ' \({\text{B}}\) ' and ' \({\text{C}}\)' reacts with each other to give dimethyl ether. Then ' \({\text{A}}\) ', ' \({\text{B}}\) ' and ' \({\text{C}}\) ' respectively are:
323016
In the following sequence of reactions, the compound \(\mathrm{Z}\) is
\[\begin{gathered}
{\text{C}}{{\text{H}}_{\text{3}}}{\text{CH = C}}{{\text{H}}_{\text{2}}}\xrightarrow[{{\text{ (ii) }}{{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}\,{\text{/}}\,{\text{OH}}}]{{{\text{(i)}}\,\,{\text{B}}{{\text{H}}_{\text{3}}}}}{\text{X}}\xrightarrow[{{\text{ - POC}}{{\text{l}}_{\text{3}}}{\text{ - HCl}}}]{{{\text{PC}}{{\text{l}}_{\text{5}}}}} \hfill \\
\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,{\text{Y}}\xrightarrow{{{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{{\text{O}}^{{\text{ - }}}}{\text{N}}{{\text{a}}^{{\text{ + }}}}}}{\text{Z}}{\text{.}} \hfill \\
\end{gathered} \]
323017
\({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}} \equiv {\text{CH}}\xrightarrow[{{\text{of}}\,{{\text{H}}_{\text{2}}}}]{{{\text{one}}\,{\text{mole}}}}{\text{(A)}}\xrightarrow{{{\text{Dry}}\,{\text{A}}{{\text{g}}_{\text{2}}}{\text{O}}}}{\text{(B)}}\)
\({\text{C}}{{\text{H}}_{\text{3}}}{\text{CH = C}}{{\text{H}}_{\text{2}}}\xrightarrow{{{\text{HCl}}}}{\text{(C)}}\xrightarrow[{{\text{Ether}}}]{{{\text{Mg}}}}{\text{(D) (B) + (D)}}\)
\(\xrightarrow{{{{\text{H}}_{\text{2}}}{\text{O}}}}{\text{(E)}}\xrightarrow[{{{\text{N}}_{\text{2}}}{\text{ + OH}}}]{{{{\text{I}}^{{\text{ + }}}}}}{\text{CH}}{{\text{I}}_{\text{3}}}{\text{(}} \downarrow {\text{) + (F)}}\xrightarrow{{{{\text{H}}_{\text{2}}}{\text{O}}}}{\text{(G)}}\)
The functional group present in compound ' G ' is
323019 Compound 'A' reacts with Na metal to give '\({\text{B}}\) '. ' \({\text{A}}\) ' also reacts with \(\mathrm{PCl}_{5}\) to give ' \({\text{C}}\) '. ' \({\text{B}}\) ' and ' \({\text{C}}\)' reacts with each other to give dimethyl ether. Then ' \({\text{A}}\) ', ' \({\text{B}}\) ' and ' \({\text{C}}\) ' respectively are: