322958
\[\begin{gathered}
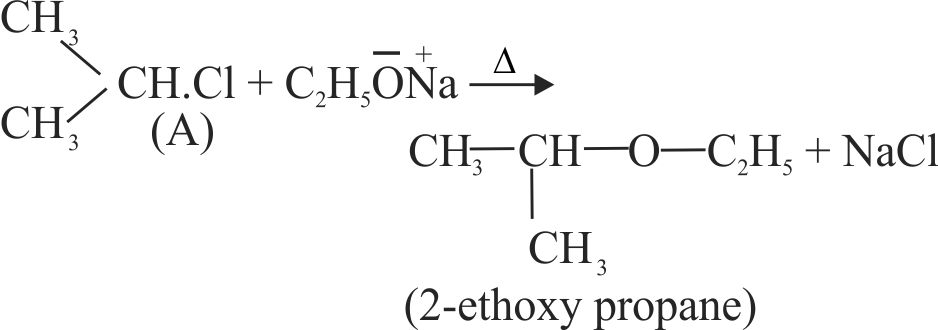
{\text{Iso}}\,{\text{ - }}\,{\text{propyl}}{\mkern 1mu} {\text{chloride + A}}\xrightarrow{\Delta } \hfill \\
\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,{\text{2 - ethoxy}}{\mkern 1mu} \,{\text{propane}}{\mkern 1mu} \,{\text{ + }}\,{\text{NaCl}} \hfill \\
\end{gathered} \].
The compound A is
322958
\[\begin{gathered}
{\text{Iso}}\,{\text{ - }}\,{\text{propyl}}{\mkern 1mu} {\text{chloride + A}}\xrightarrow{\Delta } \hfill \\
\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,{\text{2 - ethoxy}}{\mkern 1mu} \,{\text{propane}}{\mkern 1mu} \,{\text{ + }}\,{\text{NaCl}} \hfill \\
\end{gathered} \].
The compound A is
322958
\[\begin{gathered}
{\text{Iso}}\,{\text{ - }}\,{\text{propyl}}{\mkern 1mu} {\text{chloride + A}}\xrightarrow{\Delta } \hfill \\
\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,{\text{2 - ethoxy}}{\mkern 1mu} \,{\text{propane}}{\mkern 1mu} \,{\text{ + }}\,{\text{NaCl}} \hfill \\
\end{gathered} \].
The compound A is
322958
\[\begin{gathered}
{\text{Iso}}\,{\text{ - }}\,{\text{propyl}}{\mkern 1mu} {\text{chloride + A}}\xrightarrow{\Delta } \hfill \\
\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,{\text{2 - ethoxy}}{\mkern 1mu} \,{\text{propane}}{\mkern 1mu} \,{\text{ + }}\,{\text{NaCl}} \hfill \\
\end{gathered} \].
The compound A is