322320
Read Assertion and Reason carefully and mark correct option.
Assertion :
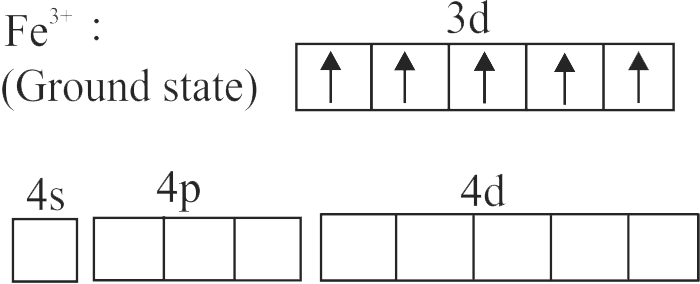
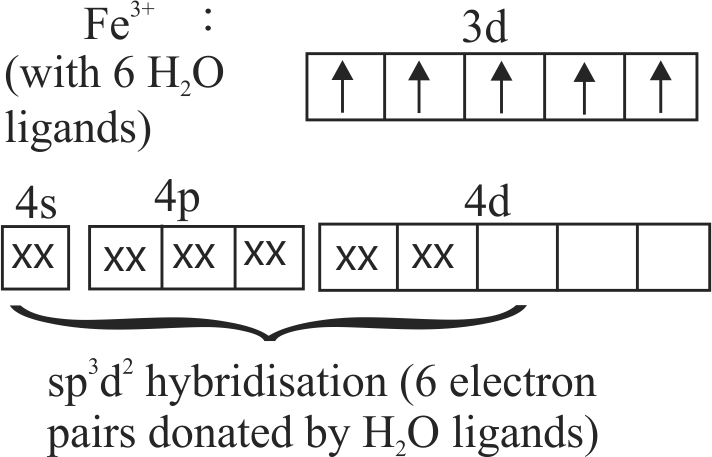
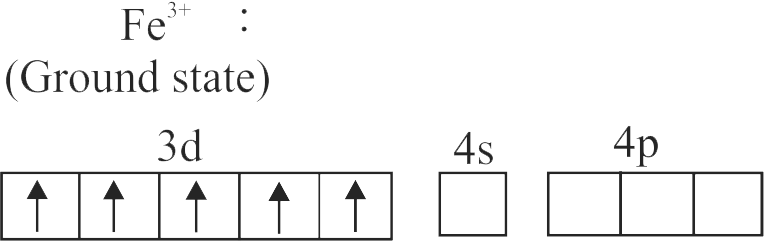
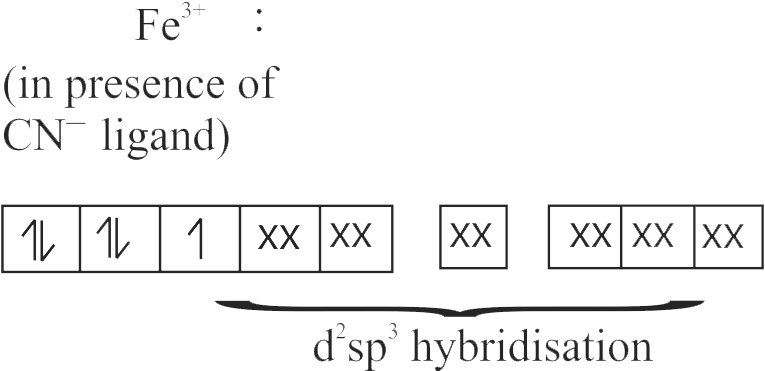
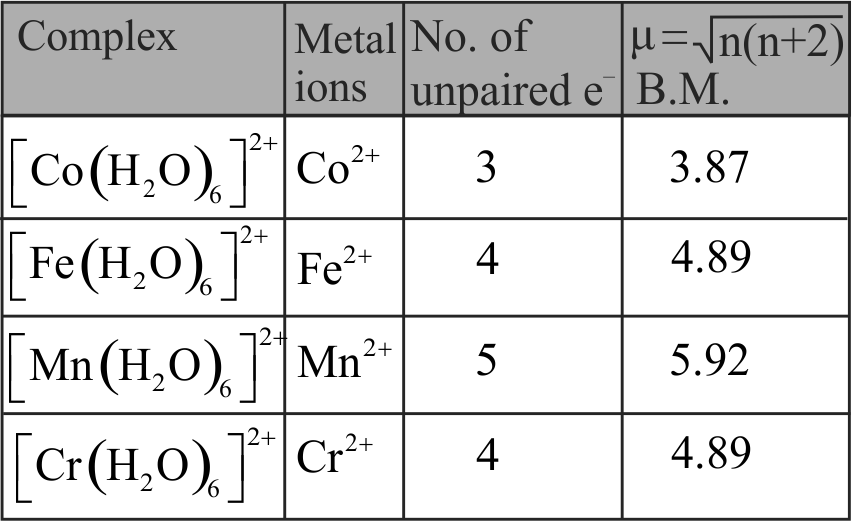
The spin only magnetic moment value for \(\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]^{3-}\) is 1.74 B.M., whereas for \(\left[\mathrm{Fe}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right]^{3+}\) is 5.92 B.M.
Reason :
In both complexes, Fe is present in \( + \,3\) oxidation state.
322320
Read Assertion and Reason carefully and mark correct option.
Assertion :
The spin only magnetic moment value for \(\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]^{3-}\) is 1.74 B.M., whereas for \(\left[\mathrm{Fe}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right]^{3+}\) is 5.92 B.M.
Reason :
In both complexes, Fe is present in \( + \,3\) oxidation state.
322320
Read Assertion and Reason carefully and mark correct option.
Assertion :
The spin only magnetic moment value for \(\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]^{3-}\) is 1.74 B.M., whereas for \(\left[\mathrm{Fe}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right]^{3+}\) is 5.92 B.M.
Reason :
In both complexes, Fe is present in \( + \,3\) oxidation state.
322320
Read Assertion and Reason carefully and mark correct option.
Assertion :
The spin only magnetic moment value for \(\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]^{3-}\) is 1.74 B.M., whereas for \(\left[\mathrm{Fe}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right]^{3+}\) is 5.92 B.M.
Reason :
In both complexes, Fe is present in \( + \,3\) oxidation state.