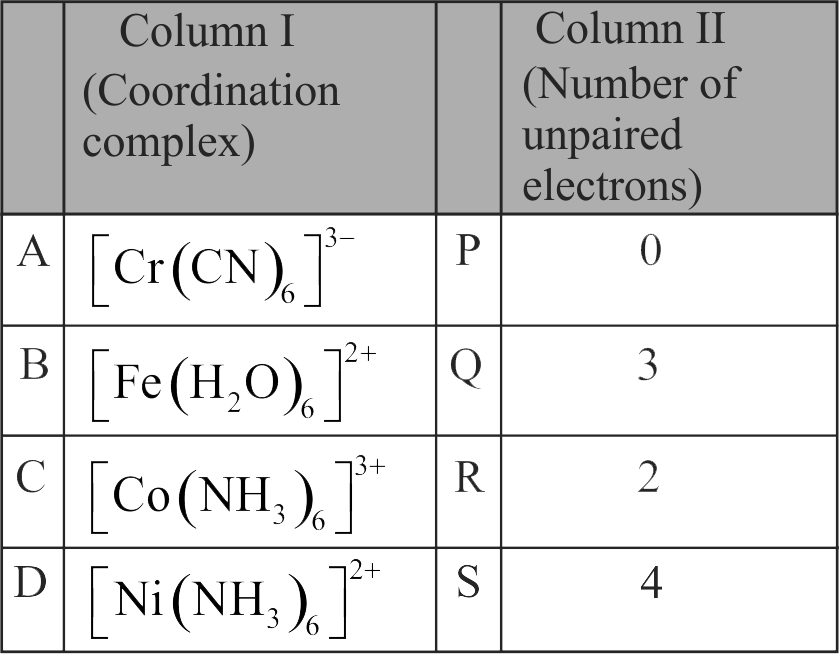
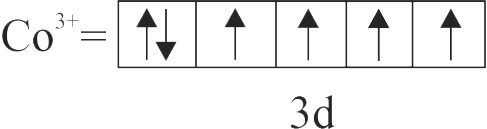
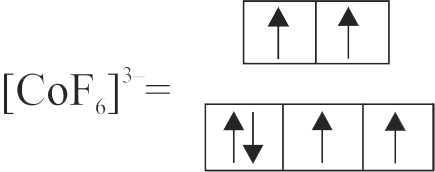
322315 The complex \(\left[\mathrm{Fe}\left(\mathrm{H}_{2} \mathrm{O}\right)_{5} \mathrm{NO}\right]^{2+}\) is formed in the brown ring test for nitrates, when freshly prepared \(\mathrm{FeSO}_{4}\) solution is added to aqueous solution of \(\mathrm{NO}_{3}^{-}\)followed by the addition of con. \(\mathrm{H}_{2} \mathrm{SO}_{4}\). Select the incorrect statement about this complex.
322315 The complex \(\left[\mathrm{Fe}\left(\mathrm{H}_{2} \mathrm{O}\right)_{5} \mathrm{NO}\right]^{2+}\) is formed in the brown ring test for nitrates, when freshly prepared \(\mathrm{FeSO}_{4}\) solution is added to aqueous solution of \(\mathrm{NO}_{3}^{-}\)followed by the addition of con. \(\mathrm{H}_{2} \mathrm{SO}_{4}\). Select the incorrect statement about this complex.
322315 The complex \(\left[\mathrm{Fe}\left(\mathrm{H}_{2} \mathrm{O}\right)_{5} \mathrm{NO}\right]^{2+}\) is formed in the brown ring test for nitrates, when freshly prepared \(\mathrm{FeSO}_{4}\) solution is added to aqueous solution of \(\mathrm{NO}_{3}^{-}\)followed by the addition of con. \(\mathrm{H}_{2} \mathrm{SO}_{4}\). Select the incorrect statement about this complex.
322315 The complex \(\left[\mathrm{Fe}\left(\mathrm{H}_{2} \mathrm{O}\right)_{5} \mathrm{NO}\right]^{2+}\) is formed in the brown ring test for nitrates, when freshly prepared \(\mathrm{FeSO}_{4}\) solution is added to aqueous solution of \(\mathrm{NO}_{3}^{-}\)followed by the addition of con. \(\mathrm{H}_{2} \mathrm{SO}_{4}\). Select the incorrect statement about this complex.
322315 The complex \(\left[\mathrm{Fe}\left(\mathrm{H}_{2} \mathrm{O}\right)_{5} \mathrm{NO}\right]^{2+}\) is formed in the brown ring test for nitrates, when freshly prepared \(\mathrm{FeSO}_{4}\) solution is added to aqueous solution of \(\mathrm{NO}_{3}^{-}\)followed by the addition of con. \(\mathrm{H}_{2} \mathrm{SO}_{4}\). Select the incorrect statement about this complex.