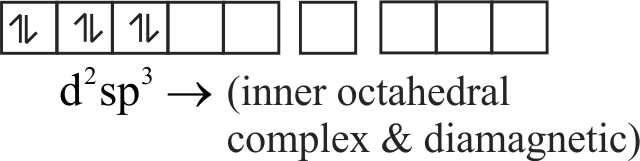322287
Which of the following is true about the complex
(i) It will have two geometrical isomeric forms, cis and trans
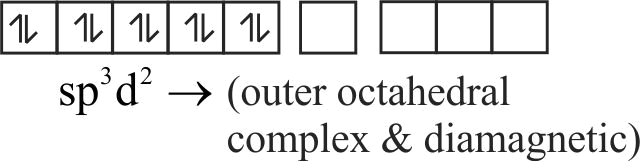
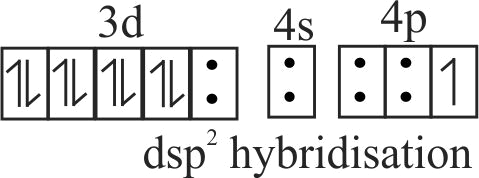
(ii) The hybridisation state of
(iii) It is a square planar complex
(iv) It is a diamagnetic complex
(v) It can show hydrate isomerism
(vi) It is a tetrahedral complex
322287
Which of the following is true about the complex
(i) It will have two geometrical isomeric forms, cis and trans
(ii) The hybridisation state of
(iii) It is a square planar complex
(iv) It is a diamagnetic complex
(v) It can show hydrate isomerism
(vi) It is a tetrahedral complex
322287
Which of the following is true about the complex
(i) It will have two geometrical isomeric forms, cis and trans
(ii) The hybridisation state of
(iii) It is a square planar complex
(iv) It is a diamagnetic complex
(v) It can show hydrate isomerism
(vi) It is a tetrahedral complex
322287
Which of the following is true about the complex
(i) It will have two geometrical isomeric forms, cis and trans
(ii) The hybridisation state of
(iii) It is a square planar complex
(iv) It is a diamagnetic complex
(v) It can show hydrate isomerism
(vi) It is a tetrahedral complex
322287
Which of the following is true about the complex
(i) It will have two geometrical isomeric forms, cis and trans
(ii) The hybridisation state of
(iii) It is a square planar complex
(iv) It is a diamagnetic complex
(v) It can show hydrate isomerism
(vi) It is a tetrahedral complex