322265
Match List - I with List - II
Column I
Column II
A
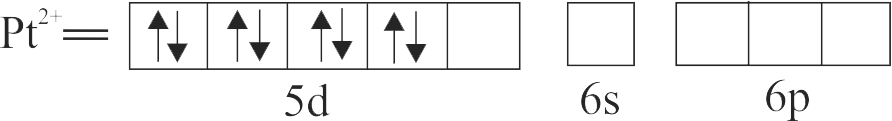
\(\left[\mathrm{PtCl}_{4}\right]^{2-}\)
P
\(\quad \mathrm{sp}^{3} \mathrm{~d}\)
B
\(\quad \mathrm{BrF}_{5}\)
Q
\(\mathrm{d}^{2} \mathrm{sp}^{3}\)
C
\(\mathrm{PCl}_{5}\)
R
\(\mathrm{dsp}^{2}\)
D
\(\left[\mathrm{Co}\left(\mathrm{NH}_{3}\right)_{6}\right]^{3+}\)
S
\(\mathrm{sp}^{3} \mathrm{~d}^{2}\)
322266
Read the Statement A and Statement B carefully to mark the correct options given below:
Statement A :
\(\operatorname{In}\left[\mathrm{Ni}(\mathrm{en})_{3}\right] \mathrm{Cl}_{2}\) has lower stability than \(\left[\mathrm{Ni}\left(\mathrm{NH}_{3}\right)_{6}\right] \mathrm{Cl}_{2}\).
Statement B :
\(\operatorname{In}\left[\mathrm{Ni}(\mathrm{en})_{3}\right] \mathrm{Cl}_{2}\), the geometry of Ni is trigonal bipyramidal.
322265
Match List - I with List - II
Column I
Column II
A
\(\left[\mathrm{PtCl}_{4}\right]^{2-}\)
P
\(\quad \mathrm{sp}^{3} \mathrm{~d}\)
B
\(\quad \mathrm{BrF}_{5}\)
Q
\(\mathrm{d}^{2} \mathrm{sp}^{3}\)
C
\(\mathrm{PCl}_{5}\)
R
\(\mathrm{dsp}^{2}\)
D
\(\left[\mathrm{Co}\left(\mathrm{NH}_{3}\right)_{6}\right]^{3+}\)
S
\(\mathrm{sp}^{3} \mathrm{~d}^{2}\)
322266
Read the Statement A and Statement B carefully to mark the correct options given below:
Statement A :
\(\operatorname{In}\left[\mathrm{Ni}(\mathrm{en})_{3}\right] \mathrm{Cl}_{2}\) has lower stability than \(\left[\mathrm{Ni}\left(\mathrm{NH}_{3}\right)_{6}\right] \mathrm{Cl}_{2}\).
Statement B :
\(\operatorname{In}\left[\mathrm{Ni}(\mathrm{en})_{3}\right] \mathrm{Cl}_{2}\), the geometry of Ni is trigonal bipyramidal.
322265
Match List - I with List - II
Column I
Column II
A
\(\left[\mathrm{PtCl}_{4}\right]^{2-}\)
P
\(\quad \mathrm{sp}^{3} \mathrm{~d}\)
B
\(\quad \mathrm{BrF}_{5}\)
Q
\(\mathrm{d}^{2} \mathrm{sp}^{3}\)
C
\(\mathrm{PCl}_{5}\)
R
\(\mathrm{dsp}^{2}\)
D
\(\left[\mathrm{Co}\left(\mathrm{NH}_{3}\right)_{6}\right]^{3+}\)
S
\(\mathrm{sp}^{3} \mathrm{~d}^{2}\)
322266
Read the Statement A and Statement B carefully to mark the correct options given below:
Statement A :
\(\operatorname{In}\left[\mathrm{Ni}(\mathrm{en})_{3}\right] \mathrm{Cl}_{2}\) has lower stability than \(\left[\mathrm{Ni}\left(\mathrm{NH}_{3}\right)_{6}\right] \mathrm{Cl}_{2}\).
Statement B :
\(\operatorname{In}\left[\mathrm{Ni}(\mathrm{en})_{3}\right] \mathrm{Cl}_{2}\), the geometry of Ni is trigonal bipyramidal.
322265
Match List - I with List - II
Column I
Column II
A
\(\left[\mathrm{PtCl}_{4}\right]^{2-}\)
P
\(\quad \mathrm{sp}^{3} \mathrm{~d}\)
B
\(\quad \mathrm{BrF}_{5}\)
Q
\(\mathrm{d}^{2} \mathrm{sp}^{3}\)
C
\(\mathrm{PCl}_{5}\)
R
\(\mathrm{dsp}^{2}\)
D
\(\left[\mathrm{Co}\left(\mathrm{NH}_{3}\right)_{6}\right]^{3+}\)
S
\(\mathrm{sp}^{3} \mathrm{~d}^{2}\)
322266
Read the Statement A and Statement B carefully to mark the correct options given below:
Statement A :
\(\operatorname{In}\left[\mathrm{Ni}(\mathrm{en})_{3}\right] \mathrm{Cl}_{2}\) has lower stability than \(\left[\mathrm{Ni}\left(\mathrm{NH}_{3}\right)_{6}\right] \mathrm{Cl}_{2}\).
Statement B :
\(\operatorname{In}\left[\mathrm{Ni}(\mathrm{en})_{3}\right] \mathrm{Cl}_{2}\), the geometry of Ni is trigonal bipyramidal.