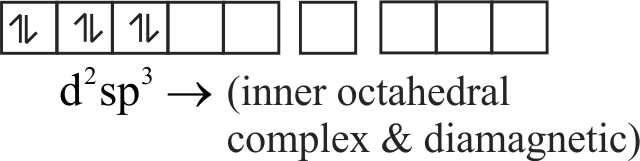322287
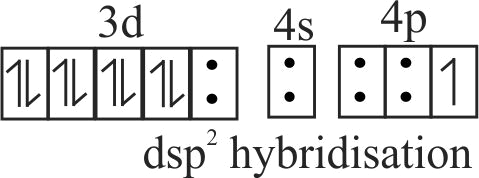
Which of the following is true about the complex \(\left[\mathrm{PtCl}_{2}\left(\mathrm{NH}_{3}\right)\left(\mathrm{OH}_{2}\right)\right]\) ? [Atomic number of \(\mathrm{Pt}=\) 78]
(i) It will have two geometrical isomeric forms, cis and trans
(ii) The hybridisation state of \(\mathrm{Pt}(\mathrm{II})\) is \(\mathrm{sp}^{3}\)
(iii) It is a square planar complex
(iv) It is a diamagnetic complex
(v) It can show hydrate isomerism
(vi) It is a tetrahedral complex
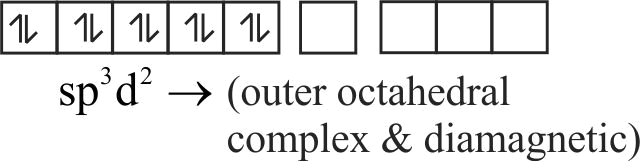
322289 Among the following complexes ( \(\mathrm{K}\) to \(\mathrm{P}\) ), \(\mathrm{K}_{3}\left[\mathrm{Fe}(\mathrm{CN})_{6}\right](\mathrm{K}),\left[\mathrm{Co}\left(\mathrm{NH}_{3}\right)_{6}\right] \mathrm{Cl}_{3}(\mathrm{~L})\), \(\mathrm{Na}_{3}\left[\mathrm{Co}(\text { oxalate })_{3}\right](\mathrm{M}),\left[\mathrm{Ni}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right] \mathrm{Cl}_{2}(\mathrm{~N})\), \(\mathrm{K}_{2}\left[\mathrm{Pt}(\mathrm{CN})_{4}\right](\mathrm{O})\), and \(\left[\mathrm{Zn}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right]\left(\mathrm{NO}_{3}\right)_{2}(\mathrm{P})\), the diamagnetic complexes are:
322287
Which of the following is true about the complex \(\left[\mathrm{PtCl}_{2}\left(\mathrm{NH}_{3}\right)\left(\mathrm{OH}_{2}\right)\right]\) ? [Atomic number of \(\mathrm{Pt}=\) 78]
(i) It will have two geometrical isomeric forms, cis and trans
(ii) The hybridisation state of \(\mathrm{Pt}(\mathrm{II})\) is \(\mathrm{sp}^{3}\)
(iii) It is a square planar complex
(iv) It is a diamagnetic complex
(v) It can show hydrate isomerism
(vi) It is a tetrahedral complex
322289 Among the following complexes ( \(\mathrm{K}\) to \(\mathrm{P}\) ), \(\mathrm{K}_{3}\left[\mathrm{Fe}(\mathrm{CN})_{6}\right](\mathrm{K}),\left[\mathrm{Co}\left(\mathrm{NH}_{3}\right)_{6}\right] \mathrm{Cl}_{3}(\mathrm{~L})\), \(\mathrm{Na}_{3}\left[\mathrm{Co}(\text { oxalate })_{3}\right](\mathrm{M}),\left[\mathrm{Ni}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right] \mathrm{Cl}_{2}(\mathrm{~N})\), \(\mathrm{K}_{2}\left[\mathrm{Pt}(\mathrm{CN})_{4}\right](\mathrm{O})\), and \(\left[\mathrm{Zn}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right]\left(\mathrm{NO}_{3}\right)_{2}(\mathrm{P})\), the diamagnetic complexes are:
322287
Which of the following is true about the complex \(\left[\mathrm{PtCl}_{2}\left(\mathrm{NH}_{3}\right)\left(\mathrm{OH}_{2}\right)\right]\) ? [Atomic number of \(\mathrm{Pt}=\) 78]
(i) It will have two geometrical isomeric forms, cis and trans
(ii) The hybridisation state of \(\mathrm{Pt}(\mathrm{II})\) is \(\mathrm{sp}^{3}\)
(iii) It is a square planar complex
(iv) It is a diamagnetic complex
(v) It can show hydrate isomerism
(vi) It is a tetrahedral complex
322289 Among the following complexes ( \(\mathrm{K}\) to \(\mathrm{P}\) ), \(\mathrm{K}_{3}\left[\mathrm{Fe}(\mathrm{CN})_{6}\right](\mathrm{K}),\left[\mathrm{Co}\left(\mathrm{NH}_{3}\right)_{6}\right] \mathrm{Cl}_{3}(\mathrm{~L})\), \(\mathrm{Na}_{3}\left[\mathrm{Co}(\text { oxalate })_{3}\right](\mathrm{M}),\left[\mathrm{Ni}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right] \mathrm{Cl}_{2}(\mathrm{~N})\), \(\mathrm{K}_{2}\left[\mathrm{Pt}(\mathrm{CN})_{4}\right](\mathrm{O})\), and \(\left[\mathrm{Zn}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right]\left(\mathrm{NO}_{3}\right)_{2}(\mathrm{P})\), the diamagnetic complexes are:
322287
Which of the following is true about the complex \(\left[\mathrm{PtCl}_{2}\left(\mathrm{NH}_{3}\right)\left(\mathrm{OH}_{2}\right)\right]\) ? [Atomic number of \(\mathrm{Pt}=\) 78]
(i) It will have two geometrical isomeric forms, cis and trans
(ii) The hybridisation state of \(\mathrm{Pt}(\mathrm{II})\) is \(\mathrm{sp}^{3}\)
(iii) It is a square planar complex
(iv) It is a diamagnetic complex
(v) It can show hydrate isomerism
(vi) It is a tetrahedral complex
322289 Among the following complexes ( \(\mathrm{K}\) to \(\mathrm{P}\) ), \(\mathrm{K}_{3}\left[\mathrm{Fe}(\mathrm{CN})_{6}\right](\mathrm{K}),\left[\mathrm{Co}\left(\mathrm{NH}_{3}\right)_{6}\right] \mathrm{Cl}_{3}(\mathrm{~L})\), \(\mathrm{Na}_{3}\left[\mathrm{Co}(\text { oxalate })_{3}\right](\mathrm{M}),\left[\mathrm{Ni}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right] \mathrm{Cl}_{2}(\mathrm{~N})\), \(\mathrm{K}_{2}\left[\mathrm{Pt}(\mathrm{CN})_{4}\right](\mathrm{O})\), and \(\left[\mathrm{Zn}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right]\left(\mathrm{NO}_{3}\right)_{2}(\mathrm{P})\), the diamagnetic complexes are:
322287
Which of the following is true about the complex \(\left[\mathrm{PtCl}_{2}\left(\mathrm{NH}_{3}\right)\left(\mathrm{OH}_{2}\right)\right]\) ? [Atomic number of \(\mathrm{Pt}=\) 78]
(i) It will have two geometrical isomeric forms, cis and trans
(ii) The hybridisation state of \(\mathrm{Pt}(\mathrm{II})\) is \(\mathrm{sp}^{3}\)
(iii) It is a square planar complex
(iv) It is a diamagnetic complex
(v) It can show hydrate isomerism
(vi) It is a tetrahedral complex
322289 Among the following complexes ( \(\mathrm{K}\) to \(\mathrm{P}\) ), \(\mathrm{K}_{3}\left[\mathrm{Fe}(\mathrm{CN})_{6}\right](\mathrm{K}),\left[\mathrm{Co}\left(\mathrm{NH}_{3}\right)_{6}\right] \mathrm{Cl}_{3}(\mathrm{~L})\), \(\mathrm{Na}_{3}\left[\mathrm{Co}(\text { oxalate })_{3}\right](\mathrm{M}),\left[\mathrm{Ni}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right] \mathrm{Cl}_{2}(\mathrm{~N})\), \(\mathrm{K}_{2}\left[\mathrm{Pt}(\mathrm{CN})_{4}\right](\mathrm{O})\), and \(\left[\mathrm{Zn}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right]\left(\mathrm{NO}_{3}\right)_{2}(\mathrm{P})\), the diamagnetic complexes are: