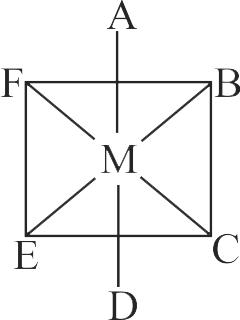
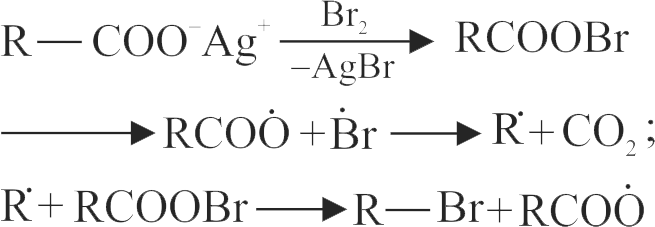321943
Among the following coordination compounds/ ions
(i) \(\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]^{3-}\)
(ii) \(\left[\mathrm{Pt}\left(\mathrm{NH}_{3}\right)_{2} \mathrm{Cl}_{2}\right]\)
(iii) \(\left[\mathrm{Co}\left(\mathrm{NH}_{3}\right)_{6}\right]^{3+}\)
(iv) \(\left[\mathrm{Cr}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right] \mathrm{Cl}_{3}\)
Which species exhibit geometrical isomerism?
321943
Among the following coordination compounds/ ions
(i) \(\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]^{3-}\)
(ii) \(\left[\mathrm{Pt}\left(\mathrm{NH}_{3}\right)_{2} \mathrm{Cl}_{2}\right]\)
(iii) \(\left[\mathrm{Co}\left(\mathrm{NH}_{3}\right)_{6}\right]^{3+}\)
(iv) \(\left[\mathrm{Cr}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right] \mathrm{Cl}_{3}\)
Which species exhibit geometrical isomerism?
321943
Among the following coordination compounds/ ions
(i) \(\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]^{3-}\)
(ii) \(\left[\mathrm{Pt}\left(\mathrm{NH}_{3}\right)_{2} \mathrm{Cl}_{2}\right]\)
(iii) \(\left[\mathrm{Co}\left(\mathrm{NH}_{3}\right)_{6}\right]^{3+}\)
(iv) \(\left[\mathrm{Cr}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right] \mathrm{Cl}_{3}\)
Which species exhibit geometrical isomerism?
321943
Among the following coordination compounds/ ions
(i) \(\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]^{3-}\)
(ii) \(\left[\mathrm{Pt}\left(\mathrm{NH}_{3}\right)_{2} \mathrm{Cl}_{2}\right]\)
(iii) \(\left[\mathrm{Co}\left(\mathrm{NH}_{3}\right)_{6}\right]^{3+}\)
(iv) \(\left[\mathrm{Cr}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right] \mathrm{Cl}_{3}\)
Which species exhibit geometrical isomerism?