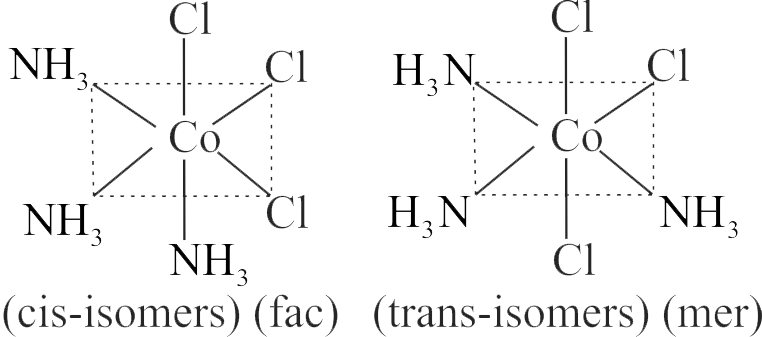
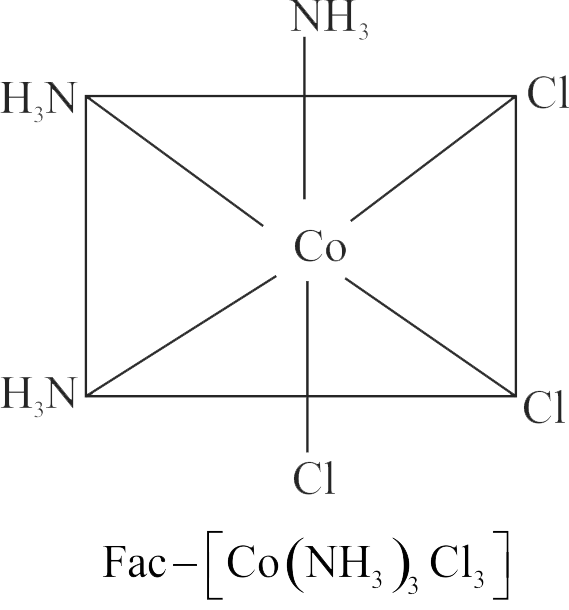
321938
Read the Assertion and Reason carefully to mark the correct option out of the options given below.
Assertion :
The total number of geometrical isomers shown by \({\mathrm{\left[\mathrm{Co}(\mathrm{en})_{2} \mathrm{Cl}_{2}\right]^{+}}}\)complex ion is three.
Reason :
\({{\rm{[Co(en}}{{\rm{)}}_{\rm{2}}}{\rm{C}}{{\rm{l}}_{\rm{2}}}{\rm{]}}^{\rm{ + }}}\) complex ion has an octahedral geometry.
321939 On treatment of \(\left[\mathrm{Pt}\left(\mathrm{NH}_{3}\right)_{4}\right]^{2+}\) with concentrated \(\mathrm{HCl}\), two compounds (I) and (II) having the same formula, \(\left[\mathrm{Pt}\left(\mathrm{NH}_{3}\right)_{2} \mathrm{Cl}_{2}\right]\) are obtained, (I) can be converted into (II) by boiling with dilute \(\mathrm{HCl}\). A solution of (I) reacts with oxalic acid to form \(\left[\mathrm{Pt}\left(\mathrm{NH}_{3}\right)_{2}\left(\mathrm{C}_{2} \mathrm{O}_{4}\right)\right]\) whereas (II) does not react. Point out the correct statement of the following.
321938
Read the Assertion and Reason carefully to mark the correct option out of the options given below.
Assertion :
The total number of geometrical isomers shown by \({\mathrm{\left[\mathrm{Co}(\mathrm{en})_{2} \mathrm{Cl}_{2}\right]^{+}}}\)complex ion is three.
Reason :
\({{\rm{[Co(en}}{{\rm{)}}_{\rm{2}}}{\rm{C}}{{\rm{l}}_{\rm{2}}}{\rm{]}}^{\rm{ + }}}\) complex ion has an octahedral geometry.
321939 On treatment of \(\left[\mathrm{Pt}\left(\mathrm{NH}_{3}\right)_{4}\right]^{2+}\) with concentrated \(\mathrm{HCl}\), two compounds (I) and (II) having the same formula, \(\left[\mathrm{Pt}\left(\mathrm{NH}_{3}\right)_{2} \mathrm{Cl}_{2}\right]\) are obtained, (I) can be converted into (II) by boiling with dilute \(\mathrm{HCl}\). A solution of (I) reacts with oxalic acid to form \(\left[\mathrm{Pt}\left(\mathrm{NH}_{3}\right)_{2}\left(\mathrm{C}_{2} \mathrm{O}_{4}\right)\right]\) whereas (II) does not react. Point out the correct statement of the following.
321938
Read the Assertion and Reason carefully to mark the correct option out of the options given below.
Assertion :
The total number of geometrical isomers shown by \({\mathrm{\left[\mathrm{Co}(\mathrm{en})_{2} \mathrm{Cl}_{2}\right]^{+}}}\)complex ion is three.
Reason :
\({{\rm{[Co(en}}{{\rm{)}}_{\rm{2}}}{\rm{C}}{{\rm{l}}_{\rm{2}}}{\rm{]}}^{\rm{ + }}}\) complex ion has an octahedral geometry.
321939 On treatment of \(\left[\mathrm{Pt}\left(\mathrm{NH}_{3}\right)_{4}\right]^{2+}\) with concentrated \(\mathrm{HCl}\), two compounds (I) and (II) having the same formula, \(\left[\mathrm{Pt}\left(\mathrm{NH}_{3}\right)_{2} \mathrm{Cl}_{2}\right]\) are obtained, (I) can be converted into (II) by boiling with dilute \(\mathrm{HCl}\). A solution of (I) reacts with oxalic acid to form \(\left[\mathrm{Pt}\left(\mathrm{NH}_{3}\right)_{2}\left(\mathrm{C}_{2} \mathrm{O}_{4}\right)\right]\) whereas (II) does not react. Point out the correct statement of the following.
321938
Read the Assertion and Reason carefully to mark the correct option out of the options given below.
Assertion :
The total number of geometrical isomers shown by \({\mathrm{\left[\mathrm{Co}(\mathrm{en})_{2} \mathrm{Cl}_{2}\right]^{+}}}\)complex ion is three.
Reason :
\({{\rm{[Co(en}}{{\rm{)}}_{\rm{2}}}{\rm{C}}{{\rm{l}}_{\rm{2}}}{\rm{]}}^{\rm{ + }}}\) complex ion has an octahedral geometry.
321939 On treatment of \(\left[\mathrm{Pt}\left(\mathrm{NH}_{3}\right)_{4}\right]^{2+}\) with concentrated \(\mathrm{HCl}\), two compounds (I) and (II) having the same formula, \(\left[\mathrm{Pt}\left(\mathrm{NH}_{3}\right)_{2} \mathrm{Cl}_{2}\right]\) are obtained, (I) can be converted into (II) by boiling with dilute \(\mathrm{HCl}\). A solution of (I) reacts with oxalic acid to form \(\left[\mathrm{Pt}\left(\mathrm{NH}_{3}\right)_{2}\left(\mathrm{C}_{2} \mathrm{O}_{4}\right)\right]\) whereas (II) does not react. Point out the correct statement of the following.
321938
Read the Assertion and Reason carefully to mark the correct option out of the options given below.
Assertion :
The total number of geometrical isomers shown by \({\mathrm{\left[\mathrm{Co}(\mathrm{en})_{2} \mathrm{Cl}_{2}\right]^{+}}}\)complex ion is three.
Reason :
\({{\rm{[Co(en}}{{\rm{)}}_{\rm{2}}}{\rm{C}}{{\rm{l}}_{\rm{2}}}{\rm{]}}^{\rm{ + }}}\) complex ion has an octahedral geometry.
321939 On treatment of \(\left[\mathrm{Pt}\left(\mathrm{NH}_{3}\right)_{4}\right]^{2+}\) with concentrated \(\mathrm{HCl}\), two compounds (I) and (II) having the same formula, \(\left[\mathrm{Pt}\left(\mathrm{NH}_{3}\right)_{2} \mathrm{Cl}_{2}\right]\) are obtained, (I) can be converted into (II) by boiling with dilute \(\mathrm{HCl}\). A solution of (I) reacts with oxalic acid to form \(\left[\mathrm{Pt}\left(\mathrm{NH}_{3}\right)_{2}\left(\mathrm{C}_{2} \mathrm{O}_{4}\right)\right]\) whereas (II) does not react. Point out the correct statement of the following.