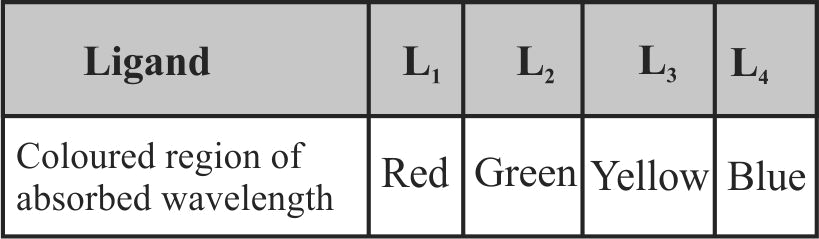
321790 The octahedral complex of a metal ion \({{\text{M}}^{{\text{3 + }}}}\) with four monodentate ligands \({{\text{L}}_{\text{1}}}{\text{,}}{{\text{L}}_{\text{2}}}{\text{,}}{{\text{L}}_{\text{3}}}\) and \({{\text{L}}_{\text{4}}}\) absorbs wavelengths in the region of red, green, yellow and blue respectively. The increasing order of ligand strength of the four ligands is:
321790 The octahedral complex of a metal ion \({{\text{M}}^{{\text{3 + }}}}\) with four monodentate ligands \({{\text{L}}_{\text{1}}}{\text{,}}{{\text{L}}_{\text{2}}}{\text{,}}{{\text{L}}_{\text{3}}}\) and \({{\text{L}}_{\text{4}}}\) absorbs wavelengths in the region of red, green, yellow and blue respectively. The increasing order of ligand strength of the four ligands is:
321790 The octahedral complex of a metal ion \({{\text{M}}^{{\text{3 + }}}}\) with four monodentate ligands \({{\text{L}}_{\text{1}}}{\text{,}}{{\text{L}}_{\text{2}}}{\text{,}}{{\text{L}}_{\text{3}}}\) and \({{\text{L}}_{\text{4}}}\) absorbs wavelengths in the region of red, green, yellow and blue respectively. The increasing order of ligand strength of the four ligands is:
321790 The octahedral complex of a metal ion \({{\text{M}}^{{\text{3 + }}}}\) with four monodentate ligands \({{\text{L}}_{\text{1}}}{\text{,}}{{\text{L}}_{\text{2}}}{\text{,}}{{\text{L}}_{\text{3}}}\) and \({{\text{L}}_{\text{4}}}\) absorbs wavelengths in the region of red, green, yellow and blue respectively. The increasing order of ligand strength of the four ligands is: