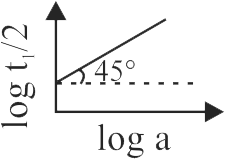
320502
The following data are for the decomposition of
ammonium nitrite in aqueous solution :
\[\begin{array}{*{20}{c}}
{{\bf{Vol}}{\bf{.}}\,{\bf{of}}\,{{\bf{N}}_{\bf{2}}}\,{\bf{in}}\,{\bf{cc}}}&{{\bf{Time(min)}}}\\
{6.25}&{10}\\
{9.00}&{15}\\
{11.40}&{20}\\
{13.65}&{25}\\
{35.65}&{{\rm{infinity}}}
\end{array}\]
The order of reaction is :
320503
Compounds '\(\mathrm{A}\)' and '\(\mathrm{B}\)' react according to the following chemical equation.
\({\text{A(g) + 2B(g)}} \to {\text{2C(g)}}\)
Concentration of either \({\text{'A'}}\,\,{\text{or}}\,\,{\text{'B'}}\) were changed keeping the concentrations of one of the reactants constant and rates were measured as a function of initial concentration. Following results were obtained. Choose the correct option for the rate equations for this reaction.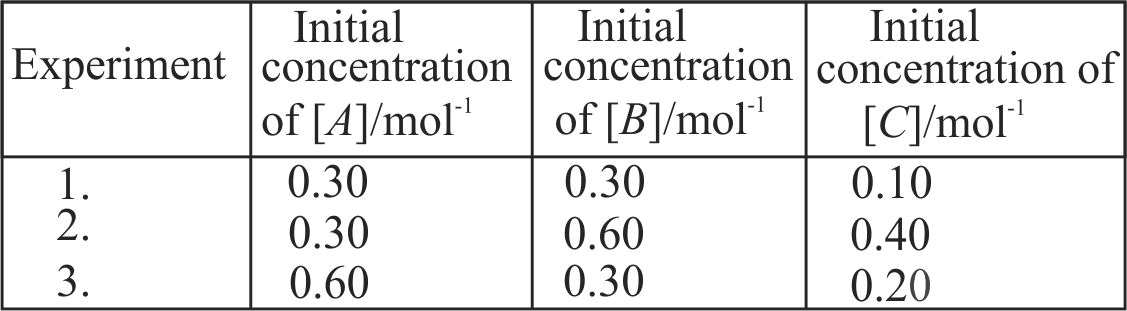
320504 For a reaction, \({\rm{A}} \to {\rm{B + C}}\), it was found that at the end of 10 minutes from the start, the total optical rotation of the system was \(50^{\circ}\) and when the reaction is complete, it was \(100^{\circ}\). Assuming that only \(\mathrm{B}\) and \(\mathrm{C}\) are optically active and dextrorotory. Calculate the rate constant of this first order reaction.
320502
The following data are for the decomposition of
ammonium nitrite in aqueous solution :
\[\begin{array}{*{20}{c}}
{{\bf{Vol}}{\bf{.}}\,{\bf{of}}\,{{\bf{N}}_{\bf{2}}}\,{\bf{in}}\,{\bf{cc}}}&{{\bf{Time(min)}}}\\
{6.25}&{10}\\
{9.00}&{15}\\
{11.40}&{20}\\
{13.65}&{25}\\
{35.65}&{{\rm{infinity}}}
\end{array}\]
The order of reaction is :
320503
Compounds '\(\mathrm{A}\)' and '\(\mathrm{B}\)' react according to the following chemical equation.
\({\text{A(g) + 2B(g)}} \to {\text{2C(g)}}\)
Concentration of either \({\text{'A'}}\,\,{\text{or}}\,\,{\text{'B'}}\) were changed keeping the concentrations of one of the reactants constant and rates were measured as a function of initial concentration. Following results were obtained. Choose the correct option for the rate equations for this reaction.
320504 For a reaction, \({\rm{A}} \to {\rm{B + C}}\), it was found that at the end of 10 minutes from the start, the total optical rotation of the system was \(50^{\circ}\) and when the reaction is complete, it was \(100^{\circ}\). Assuming that only \(\mathrm{B}\) and \(\mathrm{C}\) are optically active and dextrorotory. Calculate the rate constant of this first order reaction.
320502
The following data are for the decomposition of
ammonium nitrite in aqueous solution :
\[\begin{array}{*{20}{c}}
{{\bf{Vol}}{\bf{.}}\,{\bf{of}}\,{{\bf{N}}_{\bf{2}}}\,{\bf{in}}\,{\bf{cc}}}&{{\bf{Time(min)}}}\\
{6.25}&{10}\\
{9.00}&{15}\\
{11.40}&{20}\\
{13.65}&{25}\\
{35.65}&{{\rm{infinity}}}
\end{array}\]
The order of reaction is :
320503
Compounds '\(\mathrm{A}\)' and '\(\mathrm{B}\)' react according to the following chemical equation.
\({\text{A(g) + 2B(g)}} \to {\text{2C(g)}}\)
Concentration of either \({\text{'A'}}\,\,{\text{or}}\,\,{\text{'B'}}\) were changed keeping the concentrations of one of the reactants constant and rates were measured as a function of initial concentration. Following results were obtained. Choose the correct option for the rate equations for this reaction.
320504 For a reaction, \({\rm{A}} \to {\rm{B + C}}\), it was found that at the end of 10 minutes from the start, the total optical rotation of the system was \(50^{\circ}\) and when the reaction is complete, it was \(100^{\circ}\). Assuming that only \(\mathrm{B}\) and \(\mathrm{C}\) are optically active and dextrorotory. Calculate the rate constant of this first order reaction.
320502
The following data are for the decomposition of
ammonium nitrite in aqueous solution :
\[\begin{array}{*{20}{c}}
{{\bf{Vol}}{\bf{.}}\,{\bf{of}}\,{{\bf{N}}_{\bf{2}}}\,{\bf{in}}\,{\bf{cc}}}&{{\bf{Time(min)}}}\\
{6.25}&{10}\\
{9.00}&{15}\\
{11.40}&{20}\\
{13.65}&{25}\\
{35.65}&{{\rm{infinity}}}
\end{array}\]
The order of reaction is :
320503
Compounds '\(\mathrm{A}\)' and '\(\mathrm{B}\)' react according to the following chemical equation.
\({\text{A(g) + 2B(g)}} \to {\text{2C(g)}}\)
Concentration of either \({\text{'A'}}\,\,{\text{or}}\,\,{\text{'B'}}\) were changed keeping the concentrations of one of the reactants constant and rates were measured as a function of initial concentration. Following results were obtained. Choose the correct option for the rate equations for this reaction.
320504 For a reaction, \({\rm{A}} \to {\rm{B + C}}\), it was found that at the end of 10 minutes from the start, the total optical rotation of the system was \(50^{\circ}\) and when the reaction is complete, it was \(100^{\circ}\). Assuming that only \(\mathrm{B}\) and \(\mathrm{C}\) are optically active and dextrorotory. Calculate the rate constant of this first order reaction.