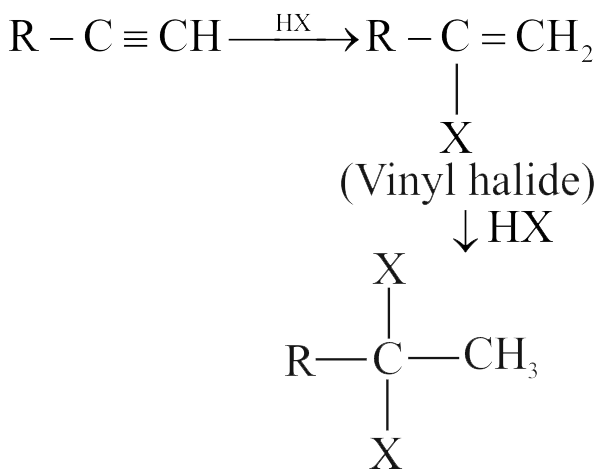
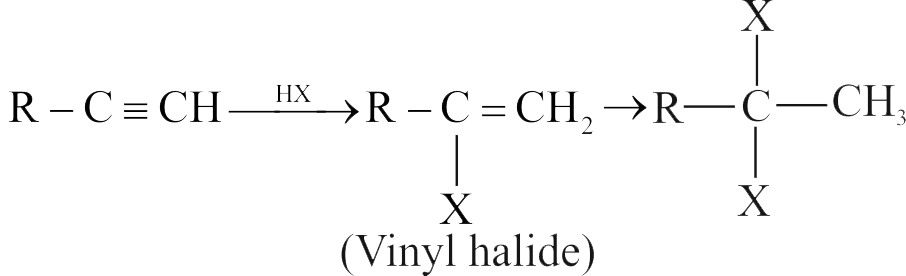
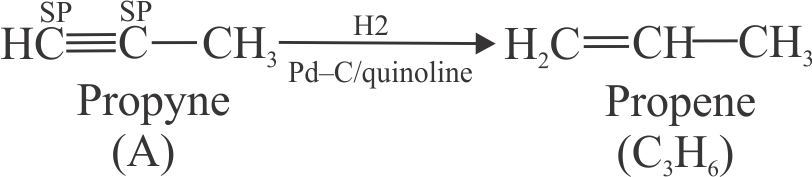
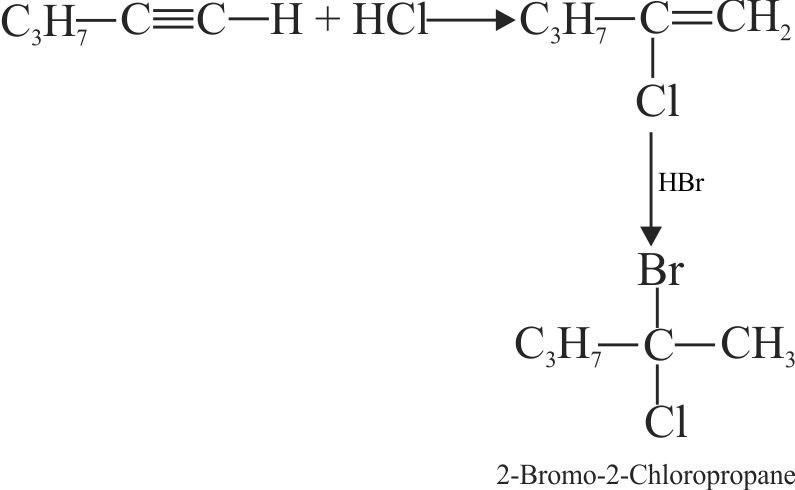
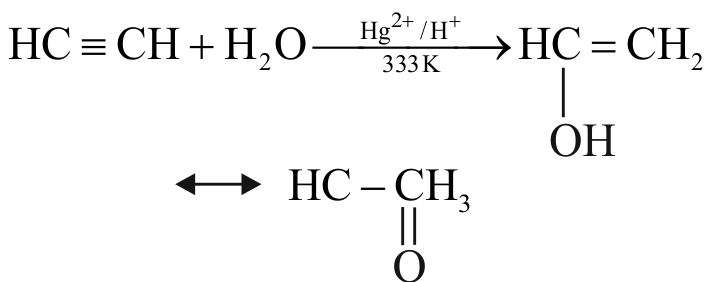
318173
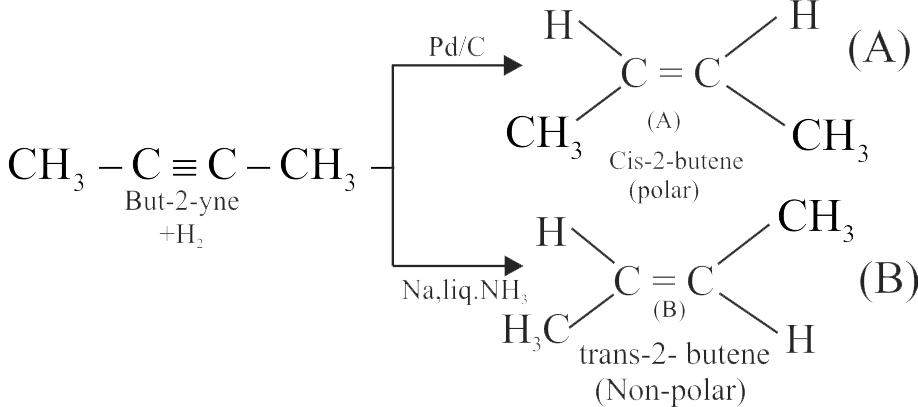
But-2-yne is reacted separately with one mole of hydrogen as shown below:
\({\text{B}}\xleftarrow[{{\text{liq}}\,\,{\text{N}}{{\text{H}}_{\text{3}}}}]{{{\text{Na}}}}{\text{C}}{{\text{H}}_{\text{3}}} - {\text{C}}\mathop \equiv \limits_{ + {{\text{H}}_2}} {\text{C}} - {\text{C}}{{\text{H}}_{\text{3}}}\xrightarrow[\Delta ]{{{\text{Pd}}/{\text{C}}}}{\text{A}}\)
1. A is more soluble than B .
2. The boiling point and melting point of \({\text{A}}\) are higher and lower than B respectively.
3. A is more polar than B because dipole moment of A is zero.
4. \(\mathrm{Br}_{2}\) adds easily to B than A .
Identify the incorrect statements from the options given below.
318173
But-2-yne is reacted separately with one mole of hydrogen as shown below:
\({\text{B}}\xleftarrow[{{\text{liq}}\,\,{\text{N}}{{\text{H}}_{\text{3}}}}]{{{\text{Na}}}}{\text{C}}{{\text{H}}_{\text{3}}} - {\text{C}}\mathop \equiv \limits_{ + {{\text{H}}_2}} {\text{C}} - {\text{C}}{{\text{H}}_{\text{3}}}\xrightarrow[\Delta ]{{{\text{Pd}}/{\text{C}}}}{\text{A}}\)
1. A is more soluble than B .
2. The boiling point and melting point of \({\text{A}}\) are higher and lower than B respectively.
3. A is more polar than B because dipole moment of A is zero.
4. \(\mathrm{Br}_{2}\) adds easily to B than A .
Identify the incorrect statements from the options given below.
318173
But-2-yne is reacted separately with one mole of hydrogen as shown below:
\({\text{B}}\xleftarrow[{{\text{liq}}\,\,{\text{N}}{{\text{H}}_{\text{3}}}}]{{{\text{Na}}}}{\text{C}}{{\text{H}}_{\text{3}}} - {\text{C}}\mathop \equiv \limits_{ + {{\text{H}}_2}} {\text{C}} - {\text{C}}{{\text{H}}_{\text{3}}}\xrightarrow[\Delta ]{{{\text{Pd}}/{\text{C}}}}{\text{A}}\)
1. A is more soluble than B .
2. The boiling point and melting point of \({\text{A}}\) are higher and lower than B respectively.
3. A is more polar than B because dipole moment of A is zero.
4. \(\mathrm{Br}_{2}\) adds easily to B than A .
Identify the incorrect statements from the options given below.
318173
But-2-yne is reacted separately with one mole of hydrogen as shown below:
\({\text{B}}\xleftarrow[{{\text{liq}}\,\,{\text{N}}{{\text{H}}_{\text{3}}}}]{{{\text{Na}}}}{\text{C}}{{\text{H}}_{\text{3}}} - {\text{C}}\mathop \equiv \limits_{ + {{\text{H}}_2}} {\text{C}} - {\text{C}}{{\text{H}}_{\text{3}}}\xrightarrow[\Delta ]{{{\text{Pd}}/{\text{C}}}}{\text{A}}\)
1. A is more soluble than B .
2. The boiling point and melting point of \({\text{A}}\) are higher and lower than B respectively.
3. A is more polar than B because dipole moment of A is zero.
4. \(\mathrm{Br}_{2}\) adds easily to B than A .
Identify the incorrect statements from the options given below.
318173
But-2-yne is reacted separately with one mole of hydrogen as shown below:
\({\text{B}}\xleftarrow[{{\text{liq}}\,\,{\text{N}}{{\text{H}}_{\text{3}}}}]{{{\text{Na}}}}{\text{C}}{{\text{H}}_{\text{3}}} - {\text{C}}\mathop \equiv \limits_{ + {{\text{H}}_2}} {\text{C}} - {\text{C}}{{\text{H}}_{\text{3}}}\xrightarrow[\Delta ]{{{\text{Pd}}/{\text{C}}}}{\text{A}}\)
1. A is more soluble than B .
2. The boiling point and melting point of \({\text{A}}\) are higher and lower than B respectively.
3. A is more polar than B because dipole moment of A is zero.
4. \(\mathrm{Br}_{2}\) adds easily to B than A .
Identify the incorrect statements from the options given below.