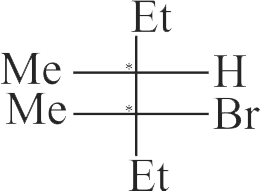
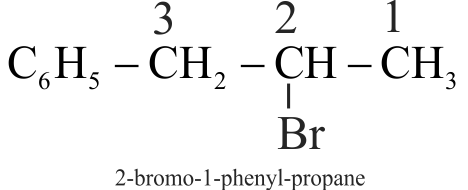318030 The intermediate carbocation formed in the reactions of \(\mathrm{HI}, \mathrm{HBr}\) and \(\mathrm{HCl}\) with propene is the same and the bond energy of \(\mathrm{HCl}, \mathrm{HBr}\) and \(\mathrm{HI}\) is \(430.5 \mathrm{~kJ} \mathrm{~mol}^{-1}, 363.7 \mathrm{~kJ} \mathrm{~mol}^{-1}\) and 296.8 \(\mathrm{kJ} \mathrm{mol}^{-1}\) respectively. What will be the order of reactivity of these halogen acids?
318030 The intermediate carbocation formed in the reactions of \(\mathrm{HI}, \mathrm{HBr}\) and \(\mathrm{HCl}\) with propene is the same and the bond energy of \(\mathrm{HCl}, \mathrm{HBr}\) and \(\mathrm{HI}\) is \(430.5 \mathrm{~kJ} \mathrm{~mol}^{-1}, 363.7 \mathrm{~kJ} \mathrm{~mol}^{-1}\) and 296.8 \(\mathrm{kJ} \mathrm{mol}^{-1}\) respectively. What will be the order of reactivity of these halogen acids?
318030 The intermediate carbocation formed in the reactions of \(\mathrm{HI}, \mathrm{HBr}\) and \(\mathrm{HCl}\) with propene is the same and the bond energy of \(\mathrm{HCl}, \mathrm{HBr}\) and \(\mathrm{HI}\) is \(430.5 \mathrm{~kJ} \mathrm{~mol}^{-1}, 363.7 \mathrm{~kJ} \mathrm{~mol}^{-1}\) and 296.8 \(\mathrm{kJ} \mathrm{mol}^{-1}\) respectively. What will be the order of reactivity of these halogen acids?
318030 The intermediate carbocation formed in the reactions of \(\mathrm{HI}, \mathrm{HBr}\) and \(\mathrm{HCl}\) with propene is the same and the bond energy of \(\mathrm{HCl}, \mathrm{HBr}\) and \(\mathrm{HI}\) is \(430.5 \mathrm{~kJ} \mathrm{~mol}^{-1}, 363.7 \mathrm{~kJ} \mathrm{~mol}^{-1}\) and 296.8 \(\mathrm{kJ} \mathrm{mol}^{-1}\) respectively. What will be the order of reactivity of these halogen acids?
318030 The intermediate carbocation formed in the reactions of \(\mathrm{HI}, \mathrm{HBr}\) and \(\mathrm{HCl}\) with propene is the same and the bond energy of \(\mathrm{HCl}, \mathrm{HBr}\) and \(\mathrm{HI}\) is \(430.5 \mathrm{~kJ} \mathrm{~mol}^{-1}, 363.7 \mathrm{~kJ} \mathrm{~mol}^{-1}\) and 296.8 \(\mathrm{kJ} \mathrm{mol}^{-1}\) respectively. What will be the order of reactivity of these halogen acids?
.jpg)
.jpg)