318038
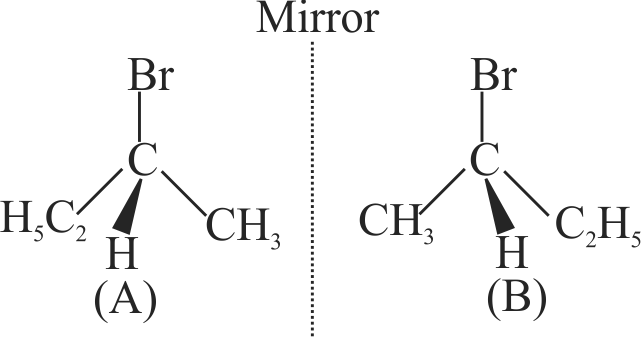
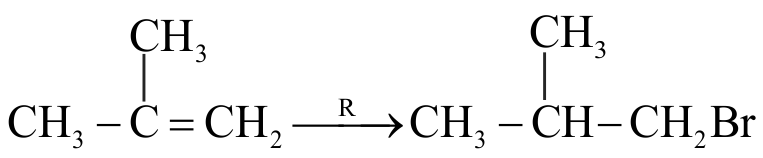
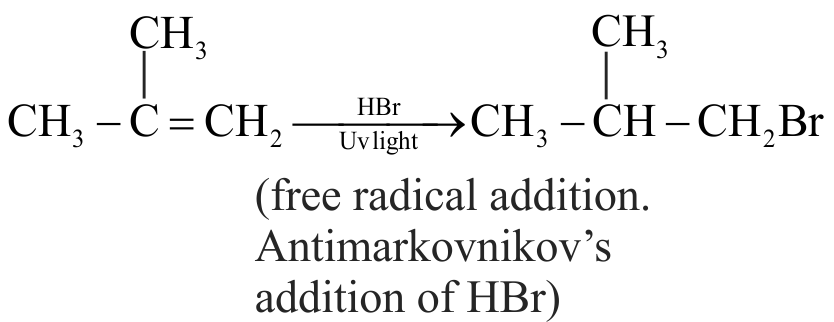
The addition of \(\mathrm{HBr}\) to 1-Butene gives a mixture of products \(\mathrm{A}, \mathrm{B}\) and \(\mathrm{C}\)
(A)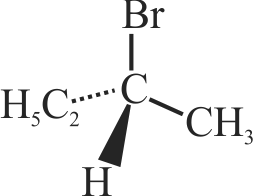
(B)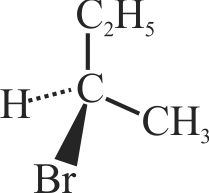
\[\begin{gathered}
{\text{C}}{{\text{H}}_{\text{3}}}{\text{ - C}}{{\text{H}}_{\text{2}}}{\text{ - C}}{{\text{H}}_{\text{2}}}{\text{ - C}}{{\text{H}}_{\text{2}}}{\text{ - Br}} \hfill \\
\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,{\text{(C)}} \hfill \\
\end{gathered} \]
The mixture consists of
318038
The addition of \(\mathrm{HBr}\) to 1-Butene gives a mixture of products \(\mathrm{A}, \mathrm{B}\) and \(\mathrm{C}\)
(A)
(B)
\[\begin{gathered}
{\text{C}}{{\text{H}}_{\text{3}}}{\text{ - C}}{{\text{H}}_{\text{2}}}{\text{ - C}}{{\text{H}}_{\text{2}}}{\text{ - C}}{{\text{H}}_{\text{2}}}{\text{ - Br}} \hfill \\
\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,{\text{(C)}} \hfill \\
\end{gathered} \]
The mixture consists of
318038
The addition of \(\mathrm{HBr}\) to 1-Butene gives a mixture of products \(\mathrm{A}, \mathrm{B}\) and \(\mathrm{C}\)
(A)
(B)
\[\begin{gathered}
{\text{C}}{{\text{H}}_{\text{3}}}{\text{ - C}}{{\text{H}}_{\text{2}}}{\text{ - C}}{{\text{H}}_{\text{2}}}{\text{ - C}}{{\text{H}}_{\text{2}}}{\text{ - Br}} \hfill \\
\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,{\text{(C)}} \hfill \\
\end{gathered} \]
The mixture consists of
318038
The addition of \(\mathrm{HBr}\) to 1-Butene gives a mixture of products \(\mathrm{A}, \mathrm{B}\) and \(\mathrm{C}\)
(A)
(B)
\[\begin{gathered}
{\text{C}}{{\text{H}}_{\text{3}}}{\text{ - C}}{{\text{H}}_{\text{2}}}{\text{ - C}}{{\text{H}}_{\text{2}}}{\text{ - C}}{{\text{H}}_{\text{2}}}{\text{ - Br}} \hfill \\
\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,{\text{(C)}} \hfill \\
\end{gathered} \]
The mixture consists of

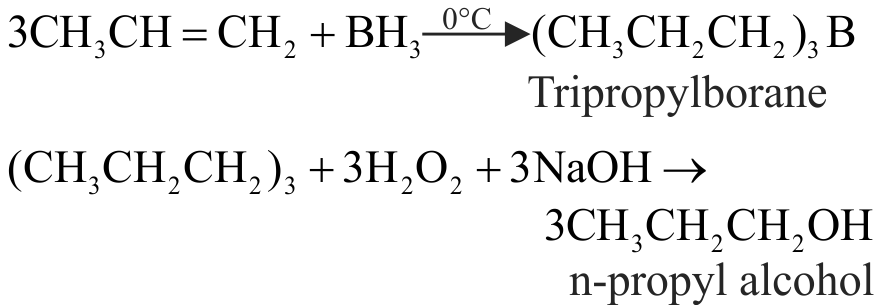
.png)