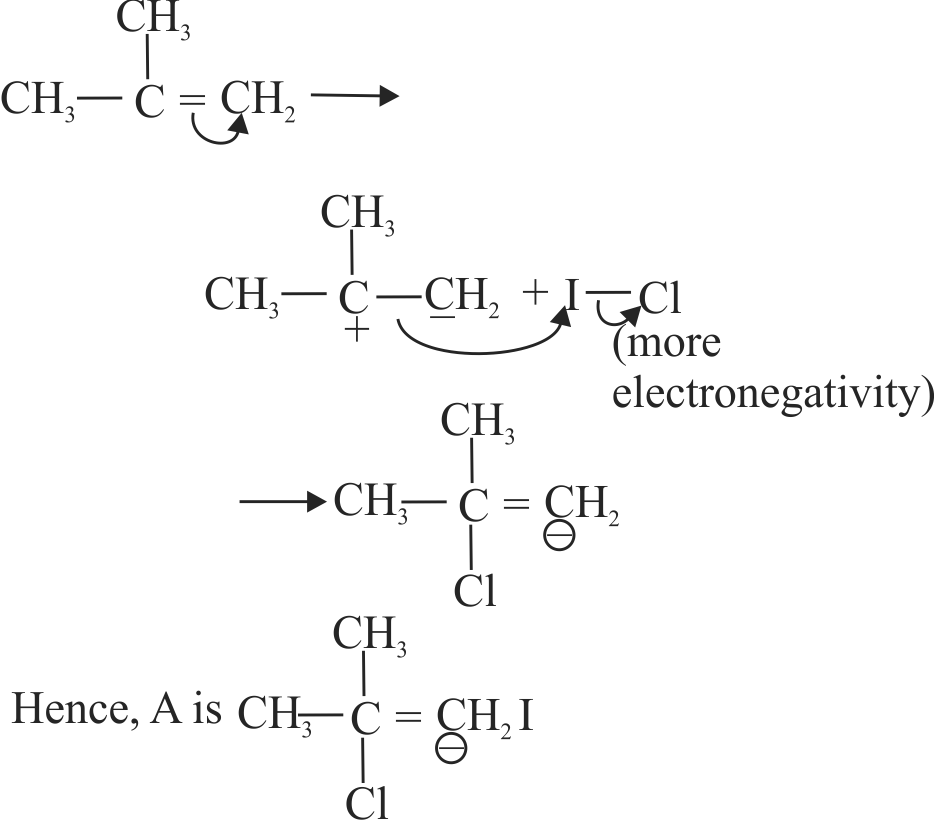
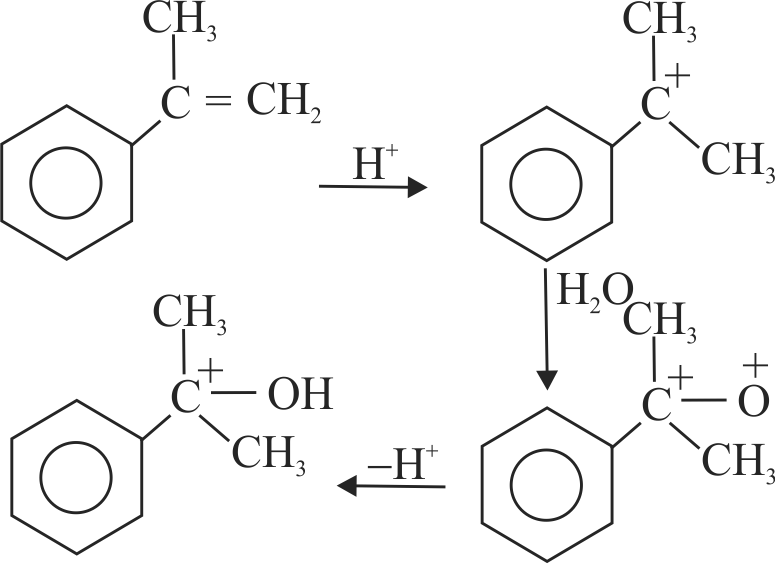
318042
Predict the major product(s) of the following reactions.
\({{\text{H}}_{\text{3}}}{\text{C - CH = C}}{{\text{H}}_{\text{2}}}\xrightarrow[{{\text{HBr}}}]{{{{{\text{(Ph - CO - O)}}}_{\text{2}}}}}\)
\({{\text{H}}_{\text{3}}}{\text{C - CH = C}}{{\text{H}}_{\text{2}}}\xrightarrow{{{\text{HBr}}}}\)
318042
Predict the major product(s) of the following reactions.
\({{\text{H}}_{\text{3}}}{\text{C - CH = C}}{{\text{H}}_{\text{2}}}\xrightarrow[{{\text{HBr}}}]{{{{{\text{(Ph - CO - O)}}}_{\text{2}}}}}\)
\({{\text{H}}_{\text{3}}}{\text{C - CH = C}}{{\text{H}}_{\text{2}}}\xrightarrow{{{\text{HBr}}}}\)
318042
Predict the major product(s) of the following reactions.
\({{\text{H}}_{\text{3}}}{\text{C - CH = C}}{{\text{H}}_{\text{2}}}\xrightarrow[{{\text{HBr}}}]{{{{{\text{(Ph - CO - O)}}}_{\text{2}}}}}\)
\({{\text{H}}_{\text{3}}}{\text{C - CH = C}}{{\text{H}}_{\text{2}}}\xrightarrow{{{\text{HBr}}}}\)
318042
Predict the major product(s) of the following reactions.
\({{\text{H}}_{\text{3}}}{\text{C - CH = C}}{{\text{H}}_{\text{2}}}\xrightarrow[{{\text{HBr}}}]{{{{{\text{(Ph - CO - O)}}}_{\text{2}}}}}\)
\({{\text{H}}_{\text{3}}}{\text{C - CH = C}}{{\text{H}}_{\text{2}}}\xrightarrow{{{\text{HBr}}}}\)