316088
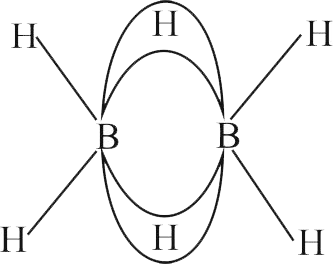
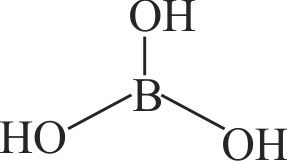
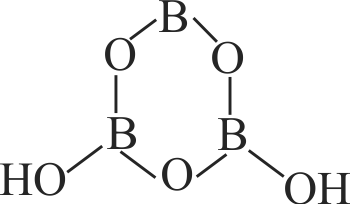
Identify the correct statement for
(A) In
(B) In
(C)
(D)
(E)
Choose the most appropriate answer from the options given below :
316088
Identify the correct statement for
(A) In
(B) In
(C)
(D)
(E)
Choose the most appropriate answer from the options given below :
316088
Identify the correct statement for
(A) In
(B) In
(C)
(D)
(E)
Choose the most appropriate answer from the options given below :
316088
Identify the correct statement for
(A) In
(B) In
(C)
(D)
(E)
Choose the most appropriate answer from the options given below :