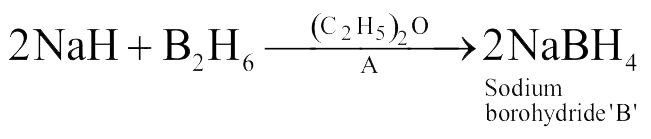316093 An alkali metal hydride \((\mathrm{NaH})\) reacts with diborane in ' \({\text{A}}\) ' to give a tetrahedral compound ' \({\text{B}}\) ' which is extensively used as reducing agent in organic synthesis. The compound ' \({\text{A}}\) ' and ' \({\text{B}}\) ' respectively are
316094
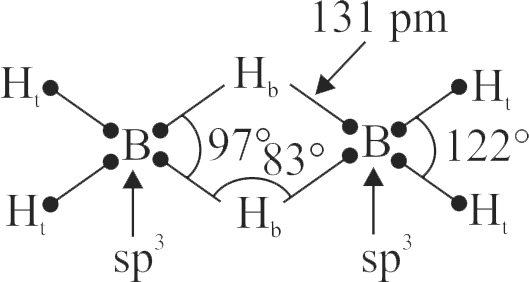
Consider the following statements for diborane:
I. Boron is approximately \(\mathrm{sp}^{3}\)-hybridized
II. \(\mathrm{B}-\mathrm{H}-\mathrm{B}\) angle is \(180^{\circ}\)
III. There are two terminal \(\mathrm{B}-\mathrm{H}\) bonds for each boron atom
IV. There are only 12 bonding electrons available. Of these statements
316093 An alkali metal hydride \((\mathrm{NaH})\) reacts with diborane in ' \({\text{A}}\) ' to give a tetrahedral compound ' \({\text{B}}\) ' which is extensively used as reducing agent in organic synthesis. The compound ' \({\text{A}}\) ' and ' \({\text{B}}\) ' respectively are
316094
Consider the following statements for diborane:
I. Boron is approximately \(\mathrm{sp}^{3}\)-hybridized
II. \(\mathrm{B}-\mathrm{H}-\mathrm{B}\) angle is \(180^{\circ}\)
III. There are two terminal \(\mathrm{B}-\mathrm{H}\) bonds for each boron atom
IV. There are only 12 bonding electrons available. Of these statements
316093 An alkali metal hydride \((\mathrm{NaH})\) reacts with diborane in ' \({\text{A}}\) ' to give a tetrahedral compound ' \({\text{B}}\) ' which is extensively used as reducing agent in organic synthesis. The compound ' \({\text{A}}\) ' and ' \({\text{B}}\) ' respectively are
316094
Consider the following statements for diborane:
I. Boron is approximately \(\mathrm{sp}^{3}\)-hybridized
II. \(\mathrm{B}-\mathrm{H}-\mathrm{B}\) angle is \(180^{\circ}\)
III. There are two terminal \(\mathrm{B}-\mathrm{H}\) bonds for each boron atom
IV. There are only 12 bonding electrons available. Of these statements
316093 An alkali metal hydride \((\mathrm{NaH})\) reacts with diborane in ' \({\text{A}}\) ' to give a tetrahedral compound ' \({\text{B}}\) ' which is extensively used as reducing agent in organic synthesis. The compound ' \({\text{A}}\) ' and ' \({\text{B}}\) ' respectively are
316094
Consider the following statements for diborane:
I. Boron is approximately \(\mathrm{sp}^{3}\)-hybridized
II. \(\mathrm{B}-\mathrm{H}-\mathrm{B}\) angle is \(180^{\circ}\)
III. There are two terminal \(\mathrm{B}-\mathrm{H}\) bonds for each boron atom
IV. There are only 12 bonding electrons available. Of these statements