316088
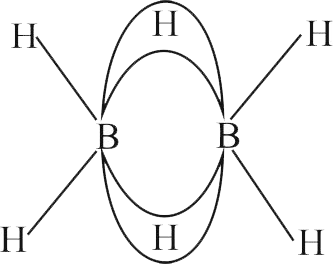
Identify the correct statement for \(\mathrm{B}_{2} \mathrm{H}_{6}\) from those given below.
(A) In \(\mathrm{B}_{2} \mathrm{H}_{6}\), all B-H bonds are equivalent.
(B) In \(\mathrm{B}_{2} \mathrm{H}_{6}\) there are four 3-centre-2-electron bonds.
(C) \(\mathrm{B}_{2} \mathrm{H}_{6}\) is a Lewis acid.
(D) \(\mathrm{B}_{2} \mathrm{H}_{6}\) can be synthesized from both \(\mathrm{BF}_{3}\) and \(\mathrm{NaBH}_{4}\).
(E) \(\mathrm{B}_{2} \mathrm{H}_{6}\) is a planar molecule.
Choose the most appropriate answer from the options given below :
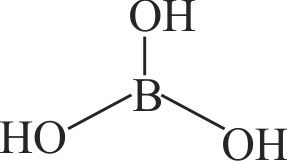
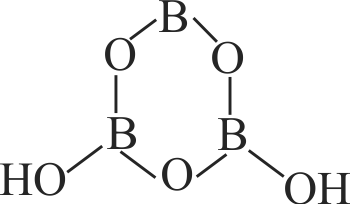
316089 A compound \(\mathrm{X}\), of boron reacts with \(\mathrm{NH}_{3}\) on heating to give another compound \(\mathrm{Y}\) which is called inroganic benzene. The compound X can be prepared by treating \(\mathrm{BF}_{3}\) with lithium aluminium hydride. The compounds \(\mathrm{X}\) and \(\mathrm{Y}\) are represented by the formulae
316088
Identify the correct statement for \(\mathrm{B}_{2} \mathrm{H}_{6}\) from those given below.
(A) In \(\mathrm{B}_{2} \mathrm{H}_{6}\), all B-H bonds are equivalent.
(B) In \(\mathrm{B}_{2} \mathrm{H}_{6}\) there are four 3-centre-2-electron bonds.
(C) \(\mathrm{B}_{2} \mathrm{H}_{6}\) is a Lewis acid.
(D) \(\mathrm{B}_{2} \mathrm{H}_{6}\) can be synthesized from both \(\mathrm{BF}_{3}\) and \(\mathrm{NaBH}_{4}\).
(E) \(\mathrm{B}_{2} \mathrm{H}_{6}\) is a planar molecule.
Choose the most appropriate answer from the options given below :
316089 A compound \(\mathrm{X}\), of boron reacts with \(\mathrm{NH}_{3}\) on heating to give another compound \(\mathrm{Y}\) which is called inroganic benzene. The compound X can be prepared by treating \(\mathrm{BF}_{3}\) with lithium aluminium hydride. The compounds \(\mathrm{X}\) and \(\mathrm{Y}\) are represented by the formulae
316088
Identify the correct statement for \(\mathrm{B}_{2} \mathrm{H}_{6}\) from those given below.
(A) In \(\mathrm{B}_{2} \mathrm{H}_{6}\), all B-H bonds are equivalent.
(B) In \(\mathrm{B}_{2} \mathrm{H}_{6}\) there are four 3-centre-2-electron bonds.
(C) \(\mathrm{B}_{2} \mathrm{H}_{6}\) is a Lewis acid.
(D) \(\mathrm{B}_{2} \mathrm{H}_{6}\) can be synthesized from both \(\mathrm{BF}_{3}\) and \(\mathrm{NaBH}_{4}\).
(E) \(\mathrm{B}_{2} \mathrm{H}_{6}\) is a planar molecule.
Choose the most appropriate answer from the options given below :
316089 A compound \(\mathrm{X}\), of boron reacts with \(\mathrm{NH}_{3}\) on heating to give another compound \(\mathrm{Y}\) which is called inroganic benzene. The compound X can be prepared by treating \(\mathrm{BF}_{3}\) with lithium aluminium hydride. The compounds \(\mathrm{X}\) and \(\mathrm{Y}\) are represented by the formulae
316088
Identify the correct statement for \(\mathrm{B}_{2} \mathrm{H}_{6}\) from those given below.
(A) In \(\mathrm{B}_{2} \mathrm{H}_{6}\), all B-H bonds are equivalent.
(B) In \(\mathrm{B}_{2} \mathrm{H}_{6}\) there are four 3-centre-2-electron bonds.
(C) \(\mathrm{B}_{2} \mathrm{H}_{6}\) is a Lewis acid.
(D) \(\mathrm{B}_{2} \mathrm{H}_{6}\) can be synthesized from both \(\mathrm{BF}_{3}\) and \(\mathrm{NaBH}_{4}\).
(E) \(\mathrm{B}_{2} \mathrm{H}_{6}\) is a planar molecule.
Choose the most appropriate answer from the options given below :
316089 A compound \(\mathrm{X}\), of boron reacts with \(\mathrm{NH}_{3}\) on heating to give another compound \(\mathrm{Y}\) which is called inroganic benzene. The compound X can be prepared by treating \(\mathrm{BF}_{3}\) with lithium aluminium hydride. The compounds \(\mathrm{X}\) and \(\mathrm{Y}\) are represented by the formulae