315583
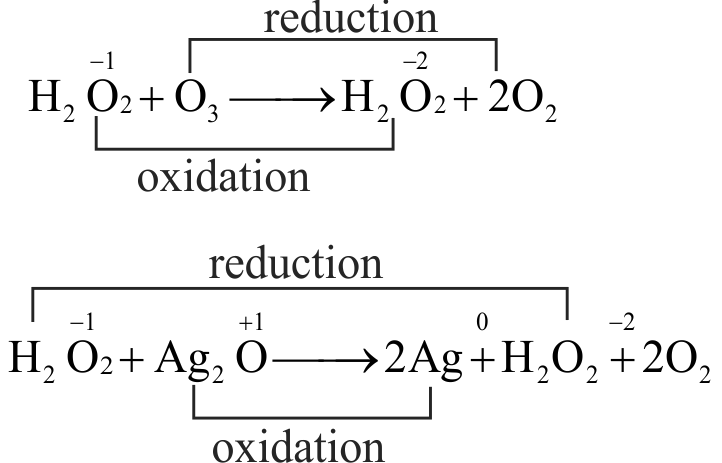
Role of hydrogen peroxide in the below reaction is respectively
\({{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}{\text{ + }}{{\text{O}}_{\text{3}}} \to {{\text{H}}_{\text{2}}}{\text{O + 2}}{{\text{O}}_{\text{2}}}\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,{\text{(i)}}\)
\({{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}{\text{ + A}}{{\text{g}}_{\text{2}}}{\text{O}} \to {\text{2Ag + }}{{\text{H}}_{\text{2}}}{\text{O + }}{{\text{O}}_{\text{2}}}\,\,\,\,\,\,\,{\text{(ii)}}\)
315583
Role of hydrogen peroxide in the below reaction is respectively
\({{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}{\text{ + }}{{\text{O}}_{\text{3}}} \to {{\text{H}}_{\text{2}}}{\text{O + 2}}{{\text{O}}_{\text{2}}}\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,{\text{(i)}}\)
\({{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}{\text{ + A}}{{\text{g}}_{\text{2}}}{\text{O}} \to {\text{2Ag + }}{{\text{H}}_{\text{2}}}{\text{O + }}{{\text{O}}_{\text{2}}}\,\,\,\,\,\,\,{\text{(ii)}}\)
315583
Role of hydrogen peroxide in the below reaction is respectively
\({{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}{\text{ + }}{{\text{O}}_{\text{3}}} \to {{\text{H}}_{\text{2}}}{\text{O + 2}}{{\text{O}}_{\text{2}}}\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,{\text{(i)}}\)
\({{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}{\text{ + A}}{{\text{g}}_{\text{2}}}{\text{O}} \to {\text{2Ag + }}{{\text{H}}_{\text{2}}}{\text{O + }}{{\text{O}}_{\text{2}}}\,\,\,\,\,\,\,{\text{(ii)}}\)
315583
Role of hydrogen peroxide in the below reaction is respectively
\({{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}{\text{ + }}{{\text{O}}_{\text{3}}} \to {{\text{H}}_{\text{2}}}{\text{O + 2}}{{\text{O}}_{\text{2}}}\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,{\text{(i)}}\)
\({{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}{\text{ + A}}{{\text{g}}_{\text{2}}}{\text{O}} \to {\text{2Ag + }}{{\text{H}}_{\text{2}}}{\text{O + }}{{\text{O}}_{\text{2}}}\,\,\,\,\,\,\,{\text{(ii)}}\)