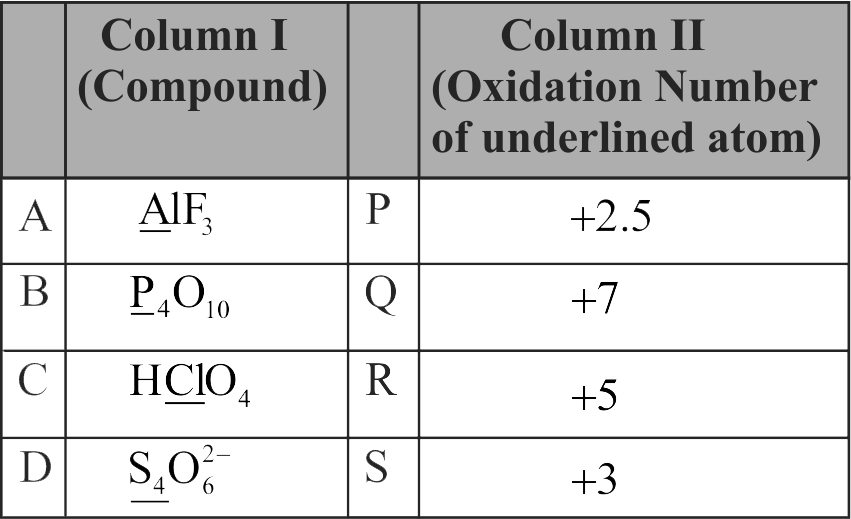
315248
Oxidation number of \(\mathrm{\mathrm{Fe}}\) in \(\mathrm{\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]^{-3}}\),
\(\mathrm{\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]^{-4},\left[\mathrm{Fe}(\mathrm{SCN})_{1}\right]^{+2}}\) and \(\mathrm{\left[\mathrm{Fe}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right]^{+3}}\) respectively would be
315248
Oxidation number of \(\mathrm{\mathrm{Fe}}\) in \(\mathrm{\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]^{-3}}\),
\(\mathrm{\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]^{-4},\left[\mathrm{Fe}(\mathrm{SCN})_{1}\right]^{+2}}\) and \(\mathrm{\left[\mathrm{Fe}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right]^{+3}}\) respectively would be
315248
Oxidation number of \(\mathrm{\mathrm{Fe}}\) in \(\mathrm{\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]^{-3}}\),
\(\mathrm{\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]^{-4},\left[\mathrm{Fe}(\mathrm{SCN})_{1}\right]^{+2}}\) and \(\mathrm{\left[\mathrm{Fe}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right]^{+3}}\) respectively would be
315248
Oxidation number of \(\mathrm{\mathrm{Fe}}\) in \(\mathrm{\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]^{-3}}\),
\(\mathrm{\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]^{-4},\left[\mathrm{Fe}(\mathrm{SCN})_{1}\right]^{+2}}\) and \(\mathrm{\left[\mathrm{Fe}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right]^{+3}}\) respectively would be