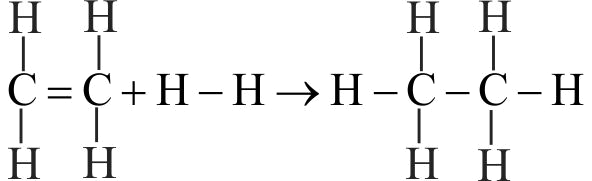369535
From the following bond energies:
\(\mathrm{H-H}\) bond energy: \(\mathrm{431.37 \mathrm{~kJ} \mathrm{~mol}^{-1}}\)
\(\mathrm{C=C}\) bond energy: \(\mathrm{606.10 \mathrm{~kJ} \mathrm{~mol}^{-1}}\)
\(\mathrm{C-C}\) bond energy: \(\mathrm{336.49 \mathrm{~kJ} \mathrm{~mol}^{-1}}\)
\(\mathrm{C-H}\) bond energy: \(\mathrm{410.50 \mathrm{~kJ} \mathrm{~mol}^{-1}}\)
Enthalpy for the reaction will be :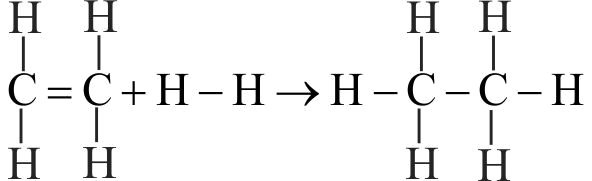
369537 The standard enthalpy of formation of \(\mathrm{\mathrm{NH}_{3}}\) is \(\mathrm{-46.0 \mathrm{~kJ} / \mathrm{mol}}\). If the enthalpy of formation of \(\mathrm{\mathrm{H}_{2}}\) from its atoms is \(\mathrm{-436 \mathrm{~kJ} / \mathrm{mol}}\) and that of \(\mathrm{N_{2}}\) is \(\mathrm{-712 \mathrm{~kJ} / \mathrm{mol}}\), the average bond enthalpy of \(\mathrm{\mathrm{N}-\mathrm{H}}\) bond in \(\mathrm{\mathrm{NH}_{3}}\) is:
369535
From the following bond energies:
\(\mathrm{H-H}\) bond energy: \(\mathrm{431.37 \mathrm{~kJ} \mathrm{~mol}^{-1}}\)
\(\mathrm{C=C}\) bond energy: \(\mathrm{606.10 \mathrm{~kJ} \mathrm{~mol}^{-1}}\)
\(\mathrm{C-C}\) bond energy: \(\mathrm{336.49 \mathrm{~kJ} \mathrm{~mol}^{-1}}\)
\(\mathrm{C-H}\) bond energy: \(\mathrm{410.50 \mathrm{~kJ} \mathrm{~mol}^{-1}}\)
Enthalpy for the reaction will be :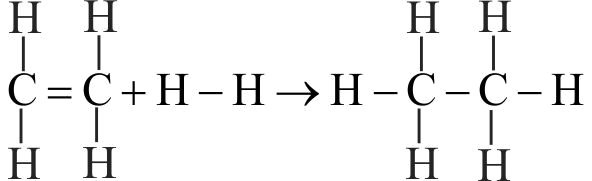
369537 The standard enthalpy of formation of \(\mathrm{\mathrm{NH}_{3}}\) is \(\mathrm{-46.0 \mathrm{~kJ} / \mathrm{mol}}\). If the enthalpy of formation of \(\mathrm{\mathrm{H}_{2}}\) from its atoms is \(\mathrm{-436 \mathrm{~kJ} / \mathrm{mol}}\) and that of \(\mathrm{N_{2}}\) is \(\mathrm{-712 \mathrm{~kJ} / \mathrm{mol}}\), the average bond enthalpy of \(\mathrm{\mathrm{N}-\mathrm{H}}\) bond in \(\mathrm{\mathrm{NH}_{3}}\) is:
369535
From the following bond energies:
\(\mathrm{H-H}\) bond energy: \(\mathrm{431.37 \mathrm{~kJ} \mathrm{~mol}^{-1}}\)
\(\mathrm{C=C}\) bond energy: \(\mathrm{606.10 \mathrm{~kJ} \mathrm{~mol}^{-1}}\)
\(\mathrm{C-C}\) bond energy: \(\mathrm{336.49 \mathrm{~kJ} \mathrm{~mol}^{-1}}\)
\(\mathrm{C-H}\) bond energy: \(\mathrm{410.50 \mathrm{~kJ} \mathrm{~mol}^{-1}}\)
Enthalpy for the reaction will be :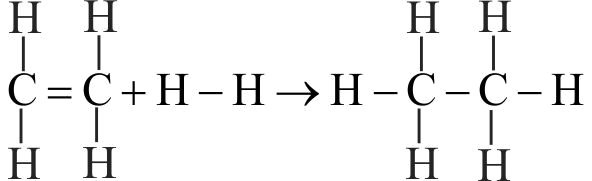
369537 The standard enthalpy of formation of \(\mathrm{\mathrm{NH}_{3}}\) is \(\mathrm{-46.0 \mathrm{~kJ} / \mathrm{mol}}\). If the enthalpy of formation of \(\mathrm{\mathrm{H}_{2}}\) from its atoms is \(\mathrm{-436 \mathrm{~kJ} / \mathrm{mol}}\) and that of \(\mathrm{N_{2}}\) is \(\mathrm{-712 \mathrm{~kJ} / \mathrm{mol}}\), the average bond enthalpy of \(\mathrm{\mathrm{N}-\mathrm{H}}\) bond in \(\mathrm{\mathrm{NH}_{3}}\) is:
369535
From the following bond energies:
\(\mathrm{H-H}\) bond energy: \(\mathrm{431.37 \mathrm{~kJ} \mathrm{~mol}^{-1}}\)
\(\mathrm{C=C}\) bond energy: \(\mathrm{606.10 \mathrm{~kJ} \mathrm{~mol}^{-1}}\)
\(\mathrm{C-C}\) bond energy: \(\mathrm{336.49 \mathrm{~kJ} \mathrm{~mol}^{-1}}\)
\(\mathrm{C-H}\) bond energy: \(\mathrm{410.50 \mathrm{~kJ} \mathrm{~mol}^{-1}}\)
Enthalpy for the reaction will be :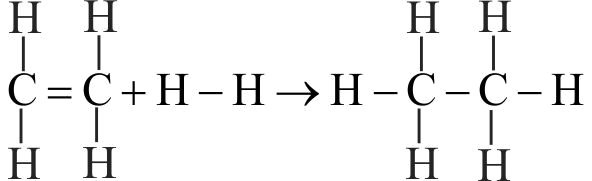
369537 The standard enthalpy of formation of \(\mathrm{\mathrm{NH}_{3}}\) is \(\mathrm{-46.0 \mathrm{~kJ} / \mathrm{mol}}\). If the enthalpy of formation of \(\mathrm{\mathrm{H}_{2}}\) from its atoms is \(\mathrm{-436 \mathrm{~kJ} / \mathrm{mol}}\) and that of \(\mathrm{N_{2}}\) is \(\mathrm{-712 \mathrm{~kJ} / \mathrm{mol}}\), the average bond enthalpy of \(\mathrm{\mathrm{N}-\mathrm{H}}\) bond in \(\mathrm{\mathrm{NH}_{3}}\) is: