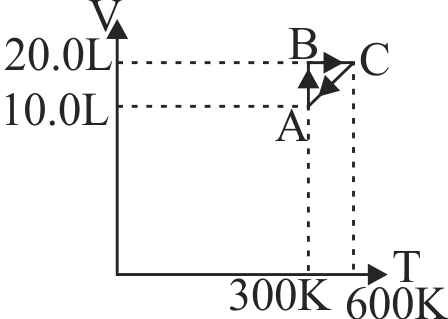
369161 The enthalpy energy change when a system goes from state \(\mathrm{A}\) to \(\mathrm{B}\) is \(\mathrm{40 \mathrm{~kJ} / \mathrm{mol}}\). If the system goes from \({\rm{B}}\,\,{\rm{to}}\,\,{\rm{A}}\) by a reversible path and returns to state A by an irreversible path what would be the change in enthalpy?
369161 The enthalpy energy change when a system goes from state \(\mathrm{A}\) to \(\mathrm{B}\) is \(\mathrm{40 \mathrm{~kJ} / \mathrm{mol}}\). If the system goes from \({\rm{B}}\,\,{\rm{to}}\,\,{\rm{A}}\) by a reversible path and returns to state A by an irreversible path what would be the change in enthalpy?
369161 The enthalpy energy change when a system goes from state \(\mathrm{A}\) to \(\mathrm{B}\) is \(\mathrm{40 \mathrm{~kJ} / \mathrm{mol}}\). If the system goes from \({\rm{B}}\,\,{\rm{to}}\,\,{\rm{A}}\) by a reversible path and returns to state A by an irreversible path what would be the change in enthalpy?
369161 The enthalpy energy change when a system goes from state \(\mathrm{A}\) to \(\mathrm{B}\) is \(\mathrm{40 \mathrm{~kJ} / \mathrm{mol}}\). If the system goes from \({\rm{B}}\,\,{\rm{to}}\,\,{\rm{A}}\) by a reversible path and returns to state A by an irreversible path what would be the change in enthalpy?