369164
Match Column I with Column II and choose the correct combination from the options given. Thermodynamic changes in gases can be expressed graphically between changes in pressure and corresponding changes in volume. Column I gives the thermodynamic change while column II shows the P - V graphs marked in serial no. 1 to 4 .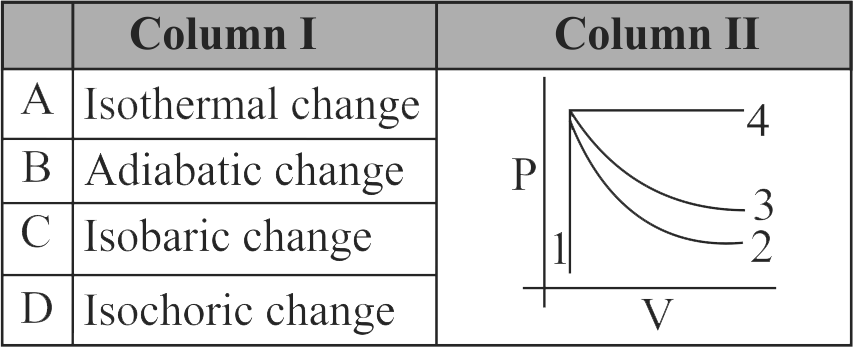
369167
Match Column I (process) with Column II (conditions).Choose the correct answer from the options given below:
Column I
Column II
A
Isothermal process
P
No heat exchange
B
Isochoric process
Q
Carried out at constant temperature
C
Isobaric process
R
Carried out at constant volume
D
Adiabatic process
S
Carried out at constant pressure
369164
Match Column I with Column II and choose the correct combination from the options given. Thermodynamic changes in gases can be expressed graphically between changes in pressure and corresponding changes in volume. Column I gives the thermodynamic change while column II shows the P - V graphs marked in serial no. 1 to 4 .
369167
Match Column I (process) with Column II (conditions).Choose the correct answer from the options given below:
Column I
Column II
A
Isothermal process
P
No heat exchange
B
Isochoric process
Q
Carried out at constant temperature
C
Isobaric process
R
Carried out at constant volume
D
Adiabatic process
S
Carried out at constant pressure
369164
Match Column I with Column II and choose the correct combination from the options given. Thermodynamic changes in gases can be expressed graphically between changes in pressure and corresponding changes in volume. Column I gives the thermodynamic change while column II shows the P - V graphs marked in serial no. 1 to 4 .
369167
Match Column I (process) with Column II (conditions).Choose the correct answer from the options given below:
Column I
Column II
A
Isothermal process
P
No heat exchange
B
Isochoric process
Q
Carried out at constant temperature
C
Isobaric process
R
Carried out at constant volume
D
Adiabatic process
S
Carried out at constant pressure
369164
Match Column I with Column II and choose the correct combination from the options given. Thermodynamic changes in gases can be expressed graphically between changes in pressure and corresponding changes in volume. Column I gives the thermodynamic change while column II shows the P - V graphs marked in serial no. 1 to 4 .
369167
Match Column I (process) with Column II (conditions).Choose the correct answer from the options given below:
Column I
Column II
A
Isothermal process
P
No heat exchange
B
Isochoric process
Q
Carried out at constant temperature
C
Isobaric process
R
Carried out at constant volume
D
Adiabatic process
S
Carried out at constant pressure
369164
Match Column I with Column II and choose the correct combination from the options given. Thermodynamic changes in gases can be expressed graphically between changes in pressure and corresponding changes in volume. Column I gives the thermodynamic change while column II shows the P - V graphs marked in serial no. 1 to 4 .
369167
Match Column I (process) with Column II (conditions).Choose the correct answer from the options given below:
Column I
Column II
A
Isothermal process
P
No heat exchange
B
Isochoric process
Q
Carried out at constant temperature
C
Isobaric process
R
Carried out at constant volume
D
Adiabatic process
S
Carried out at constant pressure
