313874
Read the Assertion and Reason carefully to mark the correct options given below:
Assertion :
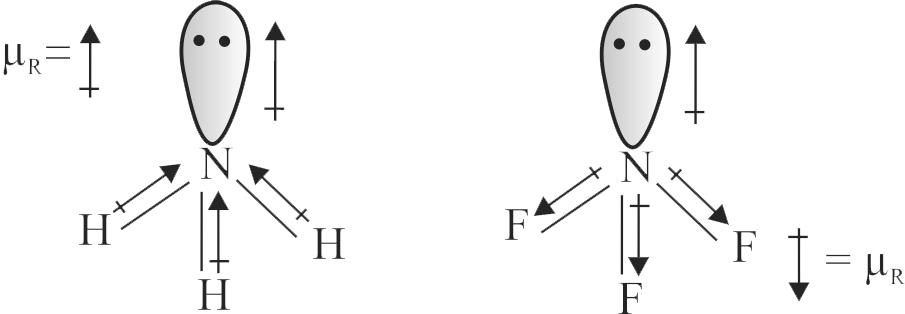
\({\mathrm{\mathrm{NH}_{3}}}\) and \({\mathrm{\mathrm{NF}_{3}}}\) molecules have pyramidal shape with a lone pair of electrons on nitrogen atom. The resultant dipole moment of \({\mathrm{\mathrm{NH}_{3}}}\) is greater than that of \({\mathrm{\mathrm{NF}_{3}}}\).
Reason :
In \({\mathrm{\mathrm{NH}_{3}}}\) ,the orbital dipole due to lone pair is in the same direction as the resultant dipole moment of the N-H bonds.F is the most electronegative element.
313874
Read the Assertion and Reason carefully to mark the correct options given below:
Assertion :
\({\mathrm{\mathrm{NH}_{3}}}\) and \({\mathrm{\mathrm{NF}_{3}}}\) molecules have pyramidal shape with a lone pair of electrons on nitrogen atom. The resultant dipole moment of \({\mathrm{\mathrm{NH}_{3}}}\) is greater than that of \({\mathrm{\mathrm{NF}_{3}}}\).
Reason :
In \({\mathrm{\mathrm{NH}_{3}}}\) ,the orbital dipole due to lone pair is in the same direction as the resultant dipole moment of the N-H bonds.F is the most electronegative element.
313874
Read the Assertion and Reason carefully to mark the correct options given below:
Assertion :
\({\mathrm{\mathrm{NH}_{3}}}\) and \({\mathrm{\mathrm{NF}_{3}}}\) molecules have pyramidal shape with a lone pair of electrons on nitrogen atom. The resultant dipole moment of \({\mathrm{\mathrm{NH}_{3}}}\) is greater than that of \({\mathrm{\mathrm{NF}_{3}}}\).
Reason :
In \({\mathrm{\mathrm{NH}_{3}}}\) ,the orbital dipole due to lone pair is in the same direction as the resultant dipole moment of the N-H bonds.F is the most electronegative element.
313874
Read the Assertion and Reason carefully to mark the correct options given below:
Assertion :
\({\mathrm{\mathrm{NH}_{3}}}\) and \({\mathrm{\mathrm{NF}_{3}}}\) molecules have pyramidal shape with a lone pair of electrons on nitrogen atom. The resultant dipole moment of \({\mathrm{\mathrm{NH}_{3}}}\) is greater than that of \({\mathrm{\mathrm{NF}_{3}}}\).
Reason :
In \({\mathrm{\mathrm{NH}_{3}}}\) ,the orbital dipole due to lone pair is in the same direction as the resultant dipole moment of the N-H bonds.F is the most electronegative element.