313870
Read the Statement \({\mathrm{A}}\) and Statement B carefully to mark the correct options given below:
Statement A :
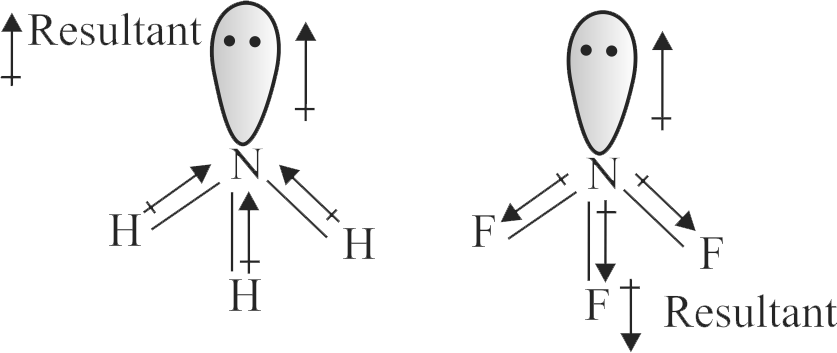
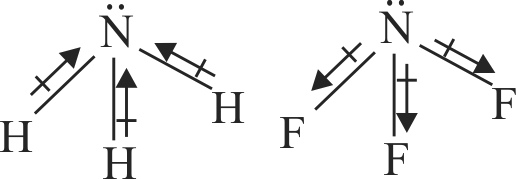
Since fluorine is more electronegative than nitrogen, the net dipole moment of \({\mathrm{\mathrm{NF}_{3}}}\) is greater than \({\mathrm{\mathrm{NH}_{3}}}\).
Statement B :
In \({\mathrm{\mathrm{NH}_{3}}}\) ,the orbital dipole due to lone pair and the dipole moment of NH bonds are in opposite direction,but in \({\mathrm{\mathrm{NF}_{3}}}\) the orbital dipole due to lone pair and dipole moments of N-F bonds are in same direction.
313872 The electronegativity difference between N and F is greater than N and H, yet the dipole moment of \({\rm{N}}{{\rm{H}}_{\rm{3}}}\left( {{\rm{1}}{\rm{.5}}\,{\rm{D}}} \right)\) is greater than that of \(N{F_3}\left( {0.2\,D} \right)\) This is because
313870
Read the Statement \({\mathrm{A}}\) and Statement B carefully to mark the correct options given below:
Statement A :
Since fluorine is more electronegative than nitrogen, the net dipole moment of \({\mathrm{\mathrm{NF}_{3}}}\) is greater than \({\mathrm{\mathrm{NH}_{3}}}\).
Statement B :
In \({\mathrm{\mathrm{NH}_{3}}}\) ,the orbital dipole due to lone pair and the dipole moment of NH bonds are in opposite direction,but in \({\mathrm{\mathrm{NF}_{3}}}\) the orbital dipole due to lone pair and dipole moments of N-F bonds are in same direction.
313872 The electronegativity difference between N and F is greater than N and H, yet the dipole moment of \({\rm{N}}{{\rm{H}}_{\rm{3}}}\left( {{\rm{1}}{\rm{.5}}\,{\rm{D}}} \right)\) is greater than that of \(N{F_3}\left( {0.2\,D} \right)\) This is because
313870
Read the Statement \({\mathrm{A}}\) and Statement B carefully to mark the correct options given below:
Statement A :
Since fluorine is more electronegative than nitrogen, the net dipole moment of \({\mathrm{\mathrm{NF}_{3}}}\) is greater than \({\mathrm{\mathrm{NH}_{3}}}\).
Statement B :
In \({\mathrm{\mathrm{NH}_{3}}}\) ,the orbital dipole due to lone pair and the dipole moment of NH bonds are in opposite direction,but in \({\mathrm{\mathrm{NF}_{3}}}\) the orbital dipole due to lone pair and dipole moments of N-F bonds are in same direction.
313872 The electronegativity difference between N and F is greater than N and H, yet the dipole moment of \({\rm{N}}{{\rm{H}}_{\rm{3}}}\left( {{\rm{1}}{\rm{.5}}\,{\rm{D}}} \right)\) is greater than that of \(N{F_3}\left( {0.2\,D} \right)\) This is because
313870
Read the Statement \({\mathrm{A}}\) and Statement B carefully to mark the correct options given below:
Statement A :
Since fluorine is more electronegative than nitrogen, the net dipole moment of \({\mathrm{\mathrm{NF}_{3}}}\) is greater than \({\mathrm{\mathrm{NH}_{3}}}\).
Statement B :
In \({\mathrm{\mathrm{NH}_{3}}}\) ,the orbital dipole due to lone pair and the dipole moment of NH bonds are in opposite direction,but in \({\mathrm{\mathrm{NF}_{3}}}\) the orbital dipole due to lone pair and dipole moments of N-F bonds are in same direction.
313872 The electronegativity difference between N and F is greater than N and H, yet the dipole moment of \({\rm{N}}{{\rm{H}}_{\rm{3}}}\left( {{\rm{1}}{\rm{.5}}\,{\rm{D}}} \right)\) is greater than that of \(N{F_3}\left( {0.2\,D} \right)\) This is because
313870
Read the Statement \({\mathrm{A}}\) and Statement B carefully to mark the correct options given below:
Statement A :
Since fluorine is more electronegative than nitrogen, the net dipole moment of \({\mathrm{\mathrm{NF}_{3}}}\) is greater than \({\mathrm{\mathrm{NH}_{3}}}\).
Statement B :
In \({\mathrm{\mathrm{NH}_{3}}}\) ,the orbital dipole due to lone pair and the dipole moment of NH bonds are in opposite direction,but in \({\mathrm{\mathrm{NF}_{3}}}\) the orbital dipole due to lone pair and dipole moments of N-F bonds are in same direction.
313872 The electronegativity difference between N and F is greater than N and H, yet the dipole moment of \({\rm{N}}{{\rm{H}}_{\rm{3}}}\left( {{\rm{1}}{\rm{.5}}\,{\rm{D}}} \right)\) is greater than that of \(N{F_3}\left( {0.2\,D} \right)\) This is because