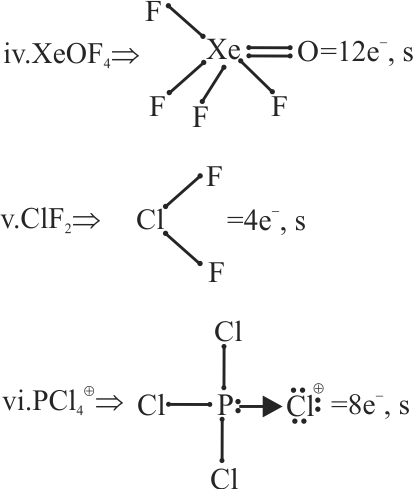313795
Read the Statement - A and Statement - B carefully to mark the correct options given below:
Statement A :
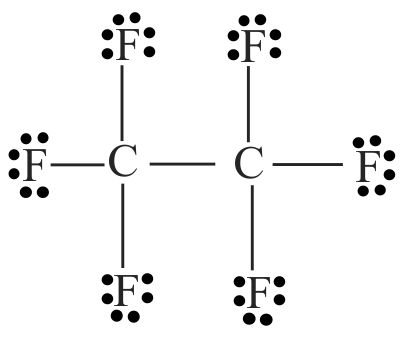
The Lewis dot structures provide a picture of bonding in molecules and ions in terms of the shared pairs of electrons and the octet rule.
Statement B :
Octet rule explains the formation of compounds of inert gases such as
313795
Read the Statement - A and Statement - B carefully to mark the correct options given below:
Statement A :
The Lewis dot structures provide a picture of bonding in molecules and ions in terms of the shared pairs of electrons and the octet rule.
Statement B :
Octet rule explains the formation of compounds of inert gases such as
313795
Read the Statement - A and Statement - B carefully to mark the correct options given below:
Statement A :
The Lewis dot structures provide a picture of bonding in molecules and ions in terms of the shared pairs of electrons and the octet rule.
Statement B :
Octet rule explains the formation of compounds of inert gases such as
313795
Read the Statement - A and Statement - B carefully to mark the correct options given below:
Statement A :
The Lewis dot structures provide a picture of bonding in molecules and ions in terms of the shared pairs of electrons and the octet rule.
Statement B :
Octet rule explains the formation of compounds of inert gases such as
313795
Read the Statement - A and Statement - B carefully to mark the correct options given below:
Statement A :
The Lewis dot structures provide a picture of bonding in molecules and ions in terms of the shared pairs of electrons and the octet rule.
Statement B :
Octet rule explains the formation of compounds of inert gases such as